Piwi
| Piwi domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
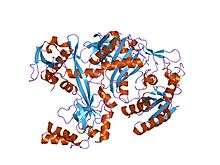 Structure of the Pyrococcus furiosus Argonaute protein.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Piwi | ||||||||
| Pfam | PF02171 | ||||||||
| InterPro | IPR003165 | ||||||||
| PROSITE | PS50822 | ||||||||
| CDD | cd02826 | ||||||||
| |||||||||
The piwi (sometimes also PIWI; originally P-element Induced WImpy testis in Drosophila[2]) class of genes was originally identified as encoding regulatory proteins responsible for maintaining incomplete differentiation in stem cells and maintaining the stability of cell division rates in germ line cells.[3] Piwi proteins are highly conserved across evolutionary lineages and are present in both plants and animals.[4] One of the major human homologues, whose upregulation is implicated in the formation of tumours such as seminomas, is called hiwi;[5] other variants on the theme include the miwi protein in mice.[6]
Role in RNA interference
The piwi domain[7] is a protein domain found in piwi proteins and a large number of related nucleic acid-binding proteins, especially those that bind and cleave RNA. The function of the domain is double stranded-RNA-guided hydrolysis of single stranded-RNA that has been determined in the argonaute family of related proteins.[1] Argonautes, the most well-studied family of nucleic-acid binding proteins, are RNase H-like enzymes that carry out the catalytic functions of the RNA-induced silencing complex (RISC). In the well-known cellular process of RNA interference, the argonaute protein in the RISC complex can bind both small interfering RNA (siRNA) generated from exogenous double-stranded RNA and microRNA (miRNA) generated from endogenous non-coding RNA, both produced by the ribonuclease Dicer, to form an RNA-RISC complex. This complex binds and cleaves complementary base pairing messenger RNA, destroying it and preventing its translation into protein. Crystallised piwi domains have a conserved basic binding site for the 5' end of bound RNA; in the case of argonaute proteins binding siRNA strands, the last unpaired nucleotide base of the siRNA is also stabilised by base stacking-interactions between the base and neighbouring tyrosine residues.[8]
Recent evidence suggests that the functional role of piwi proteins in germ-line determination is due to their capacity to interact with miRNAs. Components of the miRNA pathway appear to be present in pole plasm and to play a key role in early development and morphogenesis of Drosophila melanogaster embryos, in which germ-line maintenance has been extensively studied.[9]
piRNAs and transposon silencing
Recently, a novel class of longer-than-average miRNAs known as Piwi-interacting RNAs (piRNAs) has been defined in mammalian cells, about 26-31 nucleotides long as compared to the more typical miRNA or siRNA of about 21 nucleotides. These piRNAs are expressed mainly in spermatogenic cells in the testes of mammals.[10] However recent studies have reported that piRNA expression can be found in the ovarian somatic cells and neuron cells in invertebrates, as well as in many other mammalian somatic cells. piRNAs have been identified in the genomes of mice, rats, and humans, with an unusual "clustered" genomic organization[11] that may originate from repetitive regions of the genome such as retrotransposons or regions normally organized into heterochromatin, and which are normally derived exclusively from the antisense strand of double-stranded RNA.[12] piRNAs have thus been classified as repeat-associated small interfering RNAs (rasiRNAs).[2] Although their biogenesis is not yet well understood, piRNAs and Piwi proteins are thought to form an endogenous system for silencing the expression of selfish genetic elements such as retrotransposons and thus preventing the gene products of such sequences from interfering with germ cell formation.[12]
References
- 1 2 Rivas FV, Tolia NH, Song JJ, et al. (April 2005). "Purified Argonaute2 and an siRNA form recombinant human RISC". Nat. Struct. Mol. Biol. 12 (4): 340–9. doi:10.1038/nsmb918. PMID 15800637.
- 1 2 Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. (2006). Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev 20(16):2214-22. PMID 16882972
- ↑ Cox DN, Chao A, Lin H. (2000). piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127(3):503-14. PMID 10631171
- ↑ Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. (1998). A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev 12(23):3715-27. doi:10.1101/gad.12.23.3715 PMID 9851978
- ↑ Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H. (2002). Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene 21(25):3988-99. doi:10.1038/sj.onc.1205505 PMID 12037681
- ↑ Deng W, Lin H. (2002). miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2(6):819-30. PMID 12062093
- ↑ Cerutti L, Mian N, Bateman A (October 2000). "Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain". Trends Biochem. Sci. 25 (10): 481–2. doi:10.1016/S0968-0004(00)01641-8. PMID 11050429.
- ↑ Ma J, Yuan Y, Meister G, Pei Y, Tuschl T, Patel D (2005). "Structural basis for 5'-end-specific recognition of guide RNA by the A. fulgidus Piwi protein". Nature 434 (7033): 666-70. doi:10.1038/nature03514 PMID 15800629
- ↑ Megosh HB, Cox DN, Campbell C, Lin H. (2006). The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr Biol 16(19):1884-94. PMID 16949822
- ↑ Kim VN. (2006). Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev 20(15):1993-7. PMID 16882976
- ↑ Girard A, Sachidanandam R, Hannon GJ, Carmell MA. (2006). A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442(7099):199-202. PMID 16751776
- 1 2 Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. (2006). A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313(5785):320-4. PMID 16809489