Orotic aciduria
| Orotic aciduria | |
|---|---|
 | |
| orotic acid | |
| Classification and external resources | |
| Specialty | hematology |
| ICD-10 | D53.0 |
| ICD-9-CM | 281.4 |
| OMIM | 258900 258920 |
| DiseasesDB | 29294 |
Orotic aciduria is a disease yielding an excessive excretion of orotic acid in urine. It causes a characteristic form of anemia and may be associated with mental and physical retardation.
Orotic acid is an intermediate product in pyrimidine synthesis pathway, a subsequent product of which plays a role in conversion between dihydrofolate and tetrahydrofolate. Orotic aciduria is associated with megaloblastic anemia due to decreased pyrimidine synthesis, which leads to decreased nucleotide-lipid cofactors needed for erythrocyte membrane synthesis in the bone marrow.[1]
Signs and symptoms
In addition to the characteristic excessive orotic acid in the urine, patients typically have megaloblastic anemia (UMP synthase deficiency) which cannot be cured by administration of vitamin B12 or folic acid.[2]
It also can cause inhibition of RNA and DNA synthesis and failure to thrive.[3]
Cause and genetics
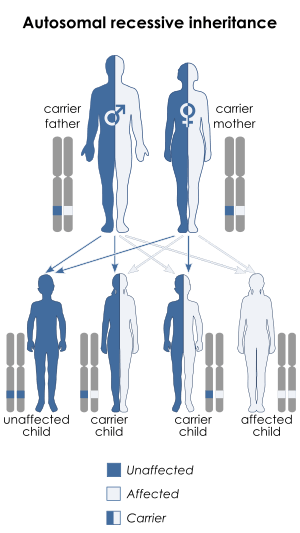
Its hereditary form, an autosomal recessive disorder,[4] can be caused by a deficiency in the enzyme UMPS,[5] a bifunctional protein that includes the enzyme activities of orotate phosphoribosyltransferase and orotidine 5'-phosphate decarboxylase.
It can also arise secondary to blockage of the urea cycle, particularly in ornithine transcarbamylase deficiency (or OTC deficiency). This can be distinguished from hereditary orotic aciduria (seen above) by assessing blood ammonia levels and blood urea nitrogen (BUN). In OTC deficiency, hyperammonemia and decreased BUN are seen because the urea cycle is not functioning properly.
Treatment
Administration of cytidine monophosphate and uridine monophosphate reduces urinary orotic acid and ameliorates the anemia.
Administration of uridine, which is converted to UMP, will bypass the metabolic block and provide the body with a source of pyrimidine.
Uridine triacetate is a drug approved by FDA to be used in the treatment of hereditary orotic aciduria.[6]
See also
References
- ↑ Balasubramaniam, S; Duley, JA; Christodoulou, J (Sep 2014). "Inborn errors of pyrimidine metabolism: clinical update and therapy.". Journal of Inherited Metabolic Disease. 37 (5): 687–98. doi:10.1007/s10545-014-9742-3. PMID 25030255.
- ↑ Huguley CM, Bain JA, Rivers SL, Scoggins RB (Jun 1959). "Refractory megaloblastic anemia associated with excretion of orotic acid". Blood. 14 (6): 615–634. PMID 13651334.
- ↑ Winkler, JK; Suttle, DP (July 1988). "Analysis of UMP synthase gene and mRNA structure in hereditary orotic aciduria fibroblasts.". American Journal of Human Genetics. 43 (1): 86–94. PMC 1715274
 . PMID 2837086.
. PMID 2837086. - ↑ Winkler JK, Suttle DP (Jul 1988). "Analysis of UMP synthase gene and mRNA structure in hereditary orotic aciduria fibroblasts". Am J Hum Genet. 43 (1): 86–94. PMC 1715274
 . PMID 2837086.
. PMID 2837086. - ↑ Suchi M, Mizuno H, Kawai Y, Tsuboi T, Sumi S, Okajima K, Hodgson ME, Ogawa H, Wada Y (Mar 1997). "Molecular cloning of the human UMP synthase gene and characterization of point mutations in two hereditary orotic aciduria families." (Free full text). American Journal of Human Genetics. 60 (3): 525–539. ISSN 0002-9297. PMC 1712531
 . PMID 9042911.
. PMID 9042911. - ↑ HIGHLIGHTS OF PRESCRIBING INFORMATION OF XURIDEN
External links
- Orotic aciduria hereditary at NIH's Office of Rare Diseases
- Orotic aciduria purines-pyrimidines at NIH's Office of Rare Diseases
- Orotic aciduria-causes, clinica -manifestations, diagnosis and treatment