Octocrylene
 | |
 | |
| Names | |
|---|---|
| IUPAC names
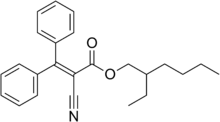
2-ethylhexyl 2-cyano-3,3- diphenyl-2-propenoate | |
| Identifiers | |
| 6197-30-4 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1201147 |
| ChemSpider | 21165 |
| ECHA InfoCard | 100.025.683 |
| PubChem | 22571 |
| UNII | 5A68WGF6WM |
| |
| |
| Properties | |
| C24H27NO2 | |
| Molar mass | 361.48 g/mol |
| Density | 1.05 g/cm3 |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 218 °C (424 °F; 491 K) at 1.5 mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Octocrylene is an organic compound used as an ingredient in sunscreens and cosmetics. It is an ester formed by the condensation of a diphenylcyanoacrylate with 2-ethylhexanol. It is a viscous, oily liquid that is clear and colorless.
The extended conjugation of the acrylate portion of the molecule absorbs UVB and short-wave UVA (ultraviolet) rays with wavelengths from 280 to 320 nm,[1] protecting the skin from direct DNA damage. The ethylhexanol portion is a fatty alcohol, adding emollient and oil-like (water resistant) properties.
This organic compound can penetrate into the skin where it acts as a photosensitizer. This results in an increased production of free radicals under illumination.[2] Free radicals are known to induce indirect DNA damage, and an increased concentration of free radicals might have contributed to the increased incidence of malignant melanoma in sunscreen-users compared to non-users (see Epidemiology of malignant melanoma). The only evidence gathered to date of a relationship between sunscreen use and malignant melanoma is correlational, but has not established a causal relationship.
See also
References
- ↑ Smart Skin Care: Octocrylene
- ↑ Hanson Kerry M.; Gratton Enrico; Bardeen Christopher J. (2006). "Sunscreen enhancement of UV-induced reactive oxygen species in the skin". Free Radical Biology and Medicine. 41 (8): 1205–1212. doi:10.1016/j.freeradbiomed.2006.06.011. PMID 17015167.