Mitochondrial fission
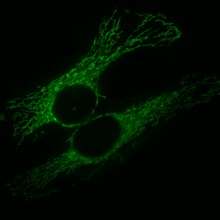
Although commonly depicted as bean-like structures, mitochondria form a highly dynamic network within most cells where they constantly undergo fission and fusion. Mitochondria can divide by prokaryotic binary fission and since they require mitochondrial DNA for their function, fission is coordinated with DNA replication. Some of the proteins that are involved in mitochondrial fission have been identified and some of them are associated with mitochondrial diseases.[1] Mitochondrial fission has significant implications in stress response and apoptosis.[2]
Mechanism
The Drp1 protein is a member of the dynamin family of large GTPases. Drp1 controls the final part of mitochondrial fission, pinching off the membrane stalk between two forming daughter mitochondria. The MFN2 protein is part of a complex that links the endoplasmic reticulum (ER) to mitochondria. Points of ER-mitochondrial association have been associated with the formation of Drp1 complexes and mitochondrial fission.[3]
Several Drp1-binding proteins have been identified.[4] A protein called mitochondrial fission factor (Mff) binds Drp1 and promotes mitochondrial fission.[5] The FIS1 protein might recruit Drp1 to sites of fission[6] but it might require association with another protein, MIEF1 (mitochondrial elongation factor 1) coded for by the SMCR7L gene, to promote mitochondrial fission. In contrast, MIEF1 when bound to Drp1 might prevent mitochondrial fission and thus shift the balance towards fusion of mitochondria.[7]
Recent research found that actin polymerization through ER-localized inverted formin 2 (INF2) was required for efficient mitochondrial fission in mammalian cells. INF2 functioned upstream of Drp1. INF2-induced actin filaments may drive initial mitochondrial constriction, which allows Drp1-driven secondary constriction.[8]
Cryo-electron tomography has been able to visualize mitochondrial division in frozen hydrated cells and has led to the suggestion that mitochondria divide by budding.[9]
See also
References
- ↑ Otera, H.; Mihara, K. (2011). "Discovery of the membrane receptor for mitochondrial fission GTPase Drp1". Small GTPases. 2 (3): 167–172. doi:10.4161/sgtp.2.3.16486. PMC 3136948
 . PMID 21776419.
. PMID 21776419. - ↑ Chan, DC (2012). "Fusion and fission: interlinked processes critical for mitochondrial health". Annu. Rev. Genet. 46: 265–287.
- ↑ Friedman, J. R.; Lackner, L. L.; West, M.; Dibenedetto, J. R.; Nunnari, J.; Voeltz, G. K. (2011). "ER Tubules Mark Sites of Mitochondrial Division". Science. 334 (6054): 358–62. doi:10.1126/science.1207385. PMC 3366560
 . PMID 21885730.
. PMID 21885730. - ↑ Dikov, D.; Reichert, A. S. (2011). "How to split up: Lessons from mitochondria". The EMBO Journal. 30 (14): 2751–2753. doi:10.1038/emboj.2011.219. PMC 3160261
 . PMID 21772324.
. PMID 21772324. - ↑ Otera, H.; Wang, C.; Cleland, M. M.; Setoguchi, K.; Yokota, S.; Youle, R. J.; Mihara, K. (2010). "Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells". The Journal of Cell Biology. 191 (6): 1141–1158. doi:10.1083/jcb.201007152. PMC 3002033
 . PMID 21149567.
. PMID 21149567. - ↑ Huang, P.; Galloway, C. A.; Yoon, Y. (2011). Jackson, Catherine L., ed. "Control of Mitochondrial Morphology Through Differential Interactions of Mitochondrial Fusion and Fission Proteins". PLoS ONE. 6 (5): e20655. doi:10.1371/journal.pone.0020655. PMC 3103587
 . PMID 21647385.
. PMID 21647385. - ↑ Zhao, J.; Liu, T.; Jin, S.; Wang, X.; Qu, M.; Uhlén, P.; Tomilin, N.; Shupliakov, O.; Lendahl, U.; Nistér, M. (2011). "Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission". The EMBO Journal. 30 (14): 2762–2778. doi:10.1038/emboj.2011.198. PMC 3160255
 . PMID 21701560.
. PMID 21701560. - ↑ Korobova, F.; Ramabhadran, V.; Higgs, H. N. (24 January 2013). "An Actin-Dependent Step in Mitochondrial Fission Mediated by the ER-Associated Formin INF2". Science. 339 (6118): 464–467. doi:10.1126/science.1228360.
- ↑ Hu, GB (May 28, 2014). "Whole Cell Cryo-electron tomography suggests mitochondria divide by budding". microsc & microanal. doi:10.1017/S1431927614001317.