Membrane vesicle trafficking
Membrane vesicle trafficking in eukaryotic animal cells involves movement of important biochemical signal molecules from synthesis-and-packaging locations in body to specific 'release' locations on the inside of the plasma membrane of the secretory cell, in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs). In this process, the 'packed' cellular products are released/secreted outside the cell across its plasma membrane. However, this vesicular membrane is retained and recycled by the secretory cells. This phenomenon has key role in synaptic neurotransmission, endocrine secretion, mucous secretion, granular-product secretion by neutrophils,etc. The scientists behind this discovery were awarded Nobel prize for the year 2013. In the prokaryotic gram-negative bacterial cells, membrane vesicle trafficking is mediated via bacterial outer membrane bounded nano-sized vesicles, called bacterial outer membrane vesicles, (OMVs)(Fig.1). In this case, however, the OMV membrane is secreted as well, along with OMV-contents to outside the secretion-active bacterium. This phenomenon has key role in host-pathogen interactions, endotoxic shock in patients, invasion and infection of animals/plants, inter-species bacterial competition, qourum sensing, exocytosis,etc.(see External Links).
Membrane vesicle trafficking in Eukaryotes
a. Intracellular trafficking occurs between subcellular compartments like Golgi cisternae and multivesicular endosomes for transport of soluble proteins as MVs.
b. Budding of MVs directly from plasma membrane as microvesicles released outside the secretory cells.
c. Exosomes are MVs that can form inside an internal compartment like multivesicular endosome. Exosomes are released eventually due to fusion of this endosome with plasma membrane of cell.
d. Hijacking of exosomal machinery by some viruses like retroviruses, wherein viruses bud inside multivesicular endosomes and get secreted subsequently as exosomes.
All these types (a-d) of modes of membrane vesicle trafficking, taking place in eukaryotic cells have been explained diagrammatically.[1]
Membrane vesicle trafficking in Prokaryotes
Unlike in eukaryotes, membrane vesicular trafficking in prokaryotes is an emerging area in interactive biology for intra-species (quorum sensing) and inter-species signaling at host-pathogen interface, as prokaryotes lack internal membrane-compartmentalization of their cytoplasm.
For more than four decades, cultures of gram negative microbes revealed the presence of nanoscale membrane vesicles. A role for membrane vesicles in pathogenic processes has been suspected since the 1970s, when they were observed in gingival plaque by electron microscopy.[2] These vesicles were suspected to promote bacterial adhesion to the host epithelial cell surface.[3] Their role in invasion of animal host cells in vivo was then demonstrated.[4] In inter-bacterial interactions, OMVs released by Pseudomonas aeruginosa microbes were shown to fuse with outer membrane of other gram negative microbes causing their bacteriolysis; these OMVs could lyse gram-positive microbes as well.[5] Role of OMVs in Helicbacter pylori infection of human primary antral epithelial cells, as model that closely resembles human stomach, has also been confirmed[6] VacA-containing OMVs could also be detected in human gastric mucosa, infected with H. pylori..[7] Salmonella OMVs were also shown to have direct role in invasion of chicken ileal epithelial cells in vivo in the year, 1993 (ref 4) and later, in hijacking of defense macrophages into sub-service for pathogen replication and consequent apoptosis of infected macrophages in typhoid-like animal infection.[8] These studies brought the focus on OMVs into membrane vesicle trafficking and showed this phenomenon as involved in multifarious processes like genetic transformation, quorum sensing, competition arsenal among microbes, etc., and invasion, infection, immuno-modulation, etc., of animal hosts.[2] A mechanism has already been proposed for generation of OMVs by gram negative microbes involving, expansion of pockets of periplasm (named, periplasmic organelles) due to accumulation of bacterial cell secretions and their pinching off as outer membrane bounded vesicles (OMVs) on the lines of a 'soap bubble' formation with a bubble tube, and further fusion or uptake of diffusing OMVs by host/target cells (Fig. 2).[9]
.png)
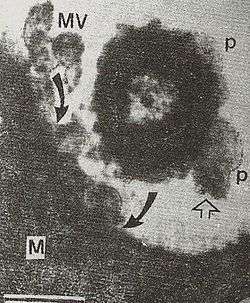
In conclusion, membrane vesicle trafficking via OMVs of Gram-negative organisms, cuts across species and kingdoms - including plant kingdom[10] - in the realm of cell-to-cell signaling.
See also
- Host-pathogen interface
- Exocytosis
- Vesicle (Biology and Chemistry)
- Bacterial outer membrane vesicles
- Virulence
- Host-pathogen interaction
- Secretory pathway
References
- ↑ Thery, C.; Ostrowsky, M.; Segura, E. (2009). "Membrane vesicles as conveyors of immuno responses". Nature Reviews Immunology. 9 (8): 581–593. doi:10.1038/nri2567.
- 1 2 Ellis, T. N.; Kuehn, M. J. (2010). "Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles". Microbiology and Molecular Biology Reviews. 74 (1): 81–94. doi:10.1128/MMBR.00031-09. ISSN 1092-2172.
- ↑ Halhoul, N.; Colvin, J.Ross (1975). "The ultrastructure of bacterial plaque attached to the gingiva of man". Archives of Oral Biology. 20 (2): 115–IN5. doi:10.1016/0003-9969(75)90164-8. ISSN 0003-9969.
- ↑ YashRoy R C (1993) Electron microscope studies of surface pili and vesicles of Salmonella 3,10:r:- organisms. Indian Journal of Animal Sciences, vol. 63(No.2), pp. 99-102.https://www.academia.edu/7327498/YashRoy_R_C_1993_Electron_microscope_studies_of_suraface_pili_and_vesicles_of_Salmonella_3_10_r_-_organisms.i_and_vesicles._Indian_Journal_of_Animal_Sciences._Vol_63_No.2_pp._99-102
- ↑ Kadurugamuwa, J.L.; Beveridge, T.J. (1996). "Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics". Journal of Bacteriology. 178 (10): 2767–2774. PMC 178010
 . PMID 8631663.
. PMID 8631663. - ↑ Heczko, U; Smith, V.C.; Meloche, R.M.; Buchan, A.M.J.; Finlay, B.B. (2000). "Characteristics of Helicobacter pylori attachment to human primary antral epithelial cells". Microbes and Infection. 2 (14): 1669–166. doi:10.1016/s1286-4579(00)01322-8. PMID 11137040.
- ↑ Fiocca, R. Neechi V. Sommi P. Ricci V. Telford J.Cover T.L.; Solcia, E. (1999). "Release of Helicobacter pylori vacuolating cytotoxin by both a specific and secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium". Journal of Pathology. 188 (2): 220–226. doi:10.1002/(sici)1096-9896(199906)188:2<220::aid-path307>3.0.co;2-c.
- ↑ YashRoy R.C. (2000) Hijacking of macrophages by Salmonella (3,10:r:-) through 'type-III' secretion-like exocytotic signaling: a mechanism for infection of chicken ileum. Indian Journal of Poultry Science, vol. 35(3), pp 276-281.https://www.researchgate.net/publication/230823526_Hijacking_of_macrophages_by_Salmonella_%28310r-%29_through_%27types-III%27_secretion-like_exocytotic_signalling_a_mechanism_for_infection_of_chicken_ileum?ev=prf_pub
- ↑ YashRoy R.C. (2003) Eucaryotic cell intoxication by Gram-negative pathogens: A novel bacterial outermembrane-bound nanovesicular exocytosis model for Type III secretion system. Toxicology International, vol. 10(1), pp. 1-9.https://www.researchgate.net/publication/230793514_Eukaryotic_cell_intoxication_by_Gram-negative_pathogens_A_novel_bacterial_outermembrane-bound_nanovesicular_exocytosis_model_for_Type-III_secretion_system._Toxicology_International_vol._10_No._1_pages_1-9_year_2003?ev=prf_pub
- ↑ Bahr O., Pruitt R., Luu D.D., Schweissinge B., Daudi A., Lui F., Ruan R., Fountaine-Bodin L., Koebnik R. and Ronald P. The Xanthomonas Ax21 protein is processed by general secretory secretion and is secreted in association with outer membrane vesicles. PeerJ picks 2014 Collection https://peerj.com/articles/242/
External links
- Nobel Prize of year 2013 in Physiology and Medicine - press release http://www.nobelprize.org/nobel_prizes/medicine/laureates/2013/press.html
- Discovery of vesicular exocytosis in prokaryotes https://www.researchgate.net/publication/230793568_Discovery_of_vesicular_exocytosis_in_prokaryotes_and_its_role_in_Salmonella_invasion?ev=prf_pub