Mechanisms of schizophrenia
The underlying mechanisms of schizophrenia, a mental disorder characterized by a disintegration of the processes of thinking and of emotional responsiveness, are complex. A number of theories attempt to explain the link between altered brain function and schizophrenia,[1] the most important of which are the dopamine hypothesis and the glutamate hypothesis. Note that these theories are separate from the causes of schizophrenia, which deal with actual starting points of the illness instead, e.g. genetic and environmental factors. The current theories attempt to explain how changes in brain functioning can contribute to symptoms of the disease.
Pathophysiology
The exact pathophysiology of schizophrenia remains poorly understood.[2] Since the coining of schizophrenia by Eugene Bleuler in 1908, quite a variety of theories have been proposed concerning its underlying pathophysiology. The most commonly supported theories are the dopamine hypothesis and the glutamate hypothesis.[3][4][5] More recent theories evolve around specific dysfunction of interneurons, abnormalities in the immune system, and oxidative stress.[6][7][8][9][10][11]
Dopamine dysfunction
Particular focus has been placed upon the function of dopamine in the mesolimbic pathway of the brain. This focus largely resulted from the accidental finding that a drug group which blocks dopamine function, known as the phenothiazines, could reduce psychotic symptoms. An influential theory, known as the "dopamine hypothesis of schizophrenia", proposes that a malfunction involving dopamine pathways is therefore the cause of (the positive symptoms of) schizophrenia. It is also supported by the fact that amphetamines, which trigger the release of dopamine, may exacerbate the psychotic symptoms in schizophrenia.[12]
This influential theory proposed that excess activation of D2 receptors is the cause of (the positive symptoms of) schizophrenia. Although postulated for about 20 years based on the D2 blockade effect common to all antipsychotics, it was not until the mid-1990s that PET and SPET imaging studies provided supporting evidence. This explanation is now thought to be simplistic, partly because newer antipsychotic medication (called atypical antipsychotic medication) can be equally effective as older medication (called typical antipsychotic medication), but also affects serotonin function and may have slightly less of a dopamine blocking effect.[13]
Evidence for this theory includes[14] findings that the potency of many antipsychotics is correlated with their affinity to dopamine D2 receptors;[15] and the exacerbatory effects of a dopamine agonist (amphetamine) and a dopamine beta hydroxylase inhibitor (disulfiram) on schizophrenia;[16][17] and post-mortem studies initially suggested increased density of dopamine D2 receptors in the striatum. Such high levels of D2 receptors intensify brain signals and can exacerbate positive symptoms (i.e. hallucinations and paranoia) in schizophrenia. Impaired glutamate (a neurotransmitter which directs neuron to pass along an impulse) activity appears to be another source of schizophrenia symptoms.[18]
However, there was controversy and conflicting findings over whether postmortem findings resulted from drug tolerance to chronic antipsychotic treatment. Compared to the success of postmortem studies in finding profound changes of dopamine receptors, imaging studies using SPET and PET methods in drug naive patients have generally failed to find any difference in dopamine D2 receptor density compared to controls. Comparable findings in longitudinal studies show: " Particular emphasis is given to methodological limitations in the existing literature, including lack of reliability data, clinical heterogeneity among studies, and inadequate study designs and statistic," suggestions are made for improving future longitudinal neuroimaging studies of treatment effects in schizophrenia[19] A recent review of imaging studies in schizophrenia shows confidence in the techniques, while discussing such operator error.[20] In 2007 one report said, "During the last decade, results of brain imaging studies by use of PET and SPET in schizophrenic patients showed a clear dysregulation of the dopaminergic system." [21]
Recent findings from meta-analyses suggest that there may be a small elevation in dopamine D2 receptors in drug-free patients with schizophrenia, but the degree of overlap between patients and controls makes it unlikely that this is clinically meaningful.[22][23] While the review by Laruelle acknowledged more sites were found using methylspiperone, it discussed the theoretical reasons behind such an increase (including the monomer-dimer equilibrium) and called for more work to be done to 'characterise' the differences. In addition, newer antipsychotic medication (called atypical antipsychotic medication) can be as potent as older medication (called typical antipsychotic medication) while also affecting serotonin function and having somewhat less of a dopamine blocking effect. In addition, dopamine pathway dysfunction has not been reliably shown to correlate with symptom onset or severity. HVA levels correlate trendwise to symptoms severity. During the application of debrisoquin, this correlation becomes significant.[24]
Giving a more precise explanation of this discrepancy in D2 receptor has been attempted by a significant minority. Radioligand imaging measurements involve the monomer and dimer ratio, and the 'cooperativity' model.[25] Cooperativitiy is a chemical function in the study of enzymes.[26] Dopamine receptors interact with their own kind, or other receptors to form higher order receptors such as dimers, via the mechanism of cooperativity.[27] Philip Seeman has said: "In schizophrenia, therefore, the density of [11C] methylspiperone sites rises, reflecting an increase in monomers, while the density of [11C] raclopride sites remains the same, indicating that the total population of D2 monomers and dimers does not change."[28] (In another place Seeman has said methylspiperone possibly binds with dimers[29]) With this difference in measurement technique in mind, the above-mentioned meta-analysis uses results from 10 different ligands.[30] Exaggerated ligand binding results such as SDZ GLC 756 (as used in the figure) were explained by reference to this monomer-dimer equilibrium.
According to Seeman, "...Numerous postmortem studies have consistently revealed D2 receptors to be elevated in the striata of patients with schizophrenia".[31] However, the authors were concerned the effect of medication may not have been fully accounted for. The study introduced an experiment by Abi-Dargham et al.[32] in which it was shown medication-free live schizophrenics had more D2 receptors involved in the schizophrenic process and more dopamine. Since then another study has shown such elevated percentages in D2 receptors is brain-wide (using a different ligand, which did not need dopamine depletion).[33][34] In a 2009 study, Annisa Abi-Dagham et al. confirmed the findings of her previous study regarding increased baseline D2 receptors in schizophrenics and showing a correlation between this magnitude and the result of amphetamine stimulation experiments.[35]
Some animal models of psychosis are similar to those for addiction – displaying increased locomotor activity.[36] For those female animals with previous sexual experience, amphetamine stimulation happens faster than for virgins. There is no study on male equivalent because the studies are meant to explain why females experience addiction earlier than males.[37]
Even in 1986 the effect of antipsychotics on receptor measurement was controversial. An article in Science sought to clarify whether the increase was solely due to medication by using drug-naive schizophrenics: "The finding that D2 dopamine receptors are substantially increased in schizophrenic patients who have never been treated with neuroleptic drugs raises the possibility that dopamine receptors are involved in the schizophrenic disease process itself. Alternatively, the increased D2 receptor number may reflect presynaptic factors such as increased endogenous dopamine levels (16). In either case, our findings support the hypothesis that dopamine receptor abnormalities are present in untreated schizophrenic patients." [38] (The experiment used 3-N-[11C]methylspiperone – the same as mentioned by Seeman detects D2 monomers and binding was double that of controls.)
It is still thought that dopamine mesolimbic pathways may be hyperactive, resulting in hyperstimulation of D2 receptors and positive symptoms. There is also growing evidence that, conversely, mesocortical pathway dopamine projections to the prefrontal cortex might be hypoactive (underactive), resulting in hypostimulation of D1 receptors, which may be related to negative symptoms and cognitive impairment. The overactivity and underactivity in these different regions may be linked, and may not be due to a primary dysfunction of dopamine systems but to more general neurodevelopmental issues that precede them.[39] Increased dopamine sensitivity may be a common final pathway.[25]
Another finding is a six-fold excess of binding sites insensitive to the testing agent, raclopride;[40][41] Seeman said this increase was probably due to the increase in D2 monomers.[28] Such an increase in monomers may occur via the cooperativity mechanism[42] which is responsible for D2High and D2Low, the supersensitive and lowsensitivity states of the D2 dopamine receptor.[43] More specifically, "an increase in monomers, may be one basis for dopamine supersensitivity".[44]
The importance of the dopamine theory has been further strengthened by the finding for common variant in the dopamine D2 receptor as candidate loci for the disease, as identified by the large genome-wide association study which included over 35.000 cases and over 110.000 controls.[45]
Exactly how dopamine dysregulation can contribute to schizophrenia symptoms remains unclear. Some studies have suggested that disruption of the auditory thalamocortical projections give rise to hallucinations,[46] while dysregulated corticostriatal circuitry and reward circuitry in the form of aberrant salience can give rise to delusions.[47]
Glutamate
Beside the dopamine hypothesis, interest has also focused on the neurotransmitter glutamate and the reduced function of the NMDA glutamate receptor in the pathophysiology of schizophrenia. This has largely been suggested by abnormally low levels of glutamate receptors found in postmortem brains of people previously diagnosed with schizophrenia[48] and the discovery that the glutamate blocking drugs such as phencyclidine and ketamine can mimic the symptoms and cognitive problems associated with the condition.[49]
The fact that reduced glutamate function is linked to poor performance on tests requiring frontal lobe and hippocampal function and that glutamate can affect dopamine function, all of which have been implicated in schizophrenia, have suggested an important mediating (and possibly causal) role of glutamate pathways in schizophrenia.[50] Positive symptoms fail however to respond to glutamatergic medication.[51] A commonly known side effect associated with schizo-affective patients known as akathisia (mistaken for schizophrenic symptoms) was found to be associated with increased levels of norepinephrine.[52]
Reduced mRNA and protein expression of several NMDA receptor subunits has also been reported in postmortem brains from patients with schizophrenia.[53]
Kynurenic acid
For the last decade increased attention has been focused on a product produced in the metabolism of the essential amino acid tryptophan in the Kynurenine pathway called kynurenic acid (KYNA). KYNA is produced from kynurenine by kynurenine aminotransferase I and II and increased levels have been observed in cerebrospinal fluid of schizophrenic patients as well as in post-mortem brains of schziphrenic patients.[54][55] KYNA has been seen to function as a NMDA receptor antagonist[56] which could explain the decreased glutamate activity seen in schizophrenic patients and goes in hand with the glutamate hypothesis of schizophrenia where NMDA receptor antagonists have been shown to induce negative symptoms of schizophrenia. Elevated levels of KYNA has also been shown to increase dopaminergic activity in midbrain[55] which in turn goes in hand with the dopamine hypothesis of schizophrenia and could explain the positive symptoms in schizophrenia. It has also been found that the mRNA levels and activity of Kynurenine 3-monooxygenase, an enzyme further down in the Kynurenine pathway, are lowered in the prefrontal cortex of schizophrenic patients[57] causing increased levels of KYNA and suggests that there might be a genetic factor involved. Beyond genetic influence, increased KYNA has also been linked to increased levels of Interleukin-1 suggesting an inflammatory component may be involved in the onset and progression of schizophrenia.[58]
Interneuron dysfunction
A more novel hypothesis concerning the pathophysiology of schizophrenia, one that closely relates to the glutamate hypothesis, is one that evolves around dysfunction of interneurons in the brain.[6][7][8] Interneurons in the brain are GABAergic, and function mainly through the inhibition of other cells.
Several studies have identified a decrease in GAD67 mRNA and protein in postmortem brains from patients diagnosed with schizophrenia as compared to controls.[59] Interestingly, these reductions were found in only a subset of cortical interneurons. Furthermore, GAD67 mRNA was completely undetectable in a subset of interneurons also expressing the calcium-binding protein parvalbumin. Levels of parvalbumin protein and mRNA were also found to be lower in patient brains in various regions in the brain. Actual numbers of parvalbumin interneurons have been found to be unchanged in these studies, however, except for a single study showing a decrease in parvalbumin interneurons in the hippocampus.[60] Together, this suggests that parvalbumin interneurons are somehow specifically affected in the disease.
Several studies have tried to assess levels in GABA in vivo in the patients with schizophrenia, but these findings have remained inconclusive.
ErbB4 protein abnormalities are also associated with neuropathophysiology of the schizophrenic brain.[61]
The largest meta-analysis on copy-number variations (CNVs), structural abnormalities in the form of tiny deletions or duplications, to date for schizophenia, published in 2015, was the first genetic evidence for the broad involvement of GABAergic neurotransmission.[62]
Immune system abnormalities
In more recent year a novel hypothesis of schizophrenia was formulated, one of inflammation and immune system abnormalities.[11] Abnormal immune system development may help explain roles of environmental effect such as prenatal hazards, post-pubertal onset, stress, climate, and infections, in addition to genetic effects.[63] The immune hypotheses is supported by findings of high levels of immune markers in the blood of schizophrenia patients.[64] High levels of immune markers have also been associated with having more severe psychotic symptoms.[65][66] Furthermore, in 2011, a meta-analysis of genome-wide association studies discovered that 129 out of 136 single-nucleotide polymorphisms (SNP) significantly associated with schizophrenia were located in the major histocompatibility complex region of the genome.[67]
Oxidative stress
A theory that has gained more support in recent years is that a large role is played in the disease by oxidative stress.[10][68] Redox dysregulation in early development can potentially influence development of different cell types that have been shown to be impaired in the disease.
Although yet unclear how exactly oxidative stress in schizophrenia would arise, oxidative stress has been shown to affect maturation of oligodendrocytes,[69] which are the myelinating cell types in the brain, possible underlying the white matter abnormalities found in the brain (see below).
Furthermore, oxidative stress could also influence the development of GABAergic interneurons,[70] which have also been found to be dysregulated in schizophrenia.
Structural abnormalities
Beside theories concerning the functional mechanism underlying the disease, quite some structural findings have been identified as well using a wide range of imaging techniques. Studies have tended to show various subtle average differences in the volume of certain areas of brain structure between people with and without diagnoses of schizophrenia, although it has become increasingly clear that there is no single pathological neuropsychological or structural neuroanatomic profile, due partly to heterogeneity within the disorder.[71]
MRI
There have also been findings of differences in the size and structure of certain brain areas in schizophrenia. A 2006 meta-analysis of MRI studies found that whole brain and hippocampal volume are reduced and that ventricular volume is increased in patients with a first psychotic episode relative to healthy controls. The average volumetric changes in these studies are however close to the limit of detection by MRI methods, so it remains to be determined whether schizophrenia is a neurodegenerative process that begins at about the time of symptom onset, or whether it is better characterised as a neurodevelopmental process that produces abnormal brain volumes at an early age.[2] In first episode psychosis typical antipsychotics like haloperidol were associated with significant reductions in gray matter volume, whereas atypical antipsychotics like olanzapine were not.[72] Studies in non-human primates found gray and white matter reductions for both typical and atypical antipsychotics.[73]
Abnormal findings in the prefrontal cortex, temporal cortex and anterior cingulate cortex are found before the first onset of schizophrenia symptoms. These regions are the regions of structural deficits found in schizophrenia and first-episode patients.[74] Positive symptoms, such as thoughts of being persecuted, were found to be related to the medial prefrontal cortex, amygdala, and hippocampus region. Negative symptoms were found to be related to the ventrolateral prefrontal cortex and ventral striatum.[74]
Ventricular and third ventricle enlargement, abnormal functioning of the amygdala, hippocampus, parahippocampal gyrus, neocortical temporal lobe regions, frontal lobe, prefontal gray matter, orbitofrontal areas, parietal lobs abnormalities and subcortical abnormalities including the cavum septi pellucidi, basal ganglia, corpus callosum, thalamus and cerebellar abnormalities. Such abnormalities usually present in the form of loss of volume.[75]
Most schizophrenia studies have found average reduced volume of the left medial temporal lobe and left superior temporal gyrus, and half of studies have revealed deficits in certain areas of the frontal gyrus, parahippocampal gyrus and temporal gyrus.[76] However, at variance with some findings in individuals with chronic schizophrenia significant group differences of temporal lobe and amygdala volumes are not shown in first-episode patients on average.[77]
Finally, MRI studies utilizing modern cortical surface reconstruction techniques have shown widespread reduction in cerebral cortical thickness (i.e., "cortical thinning") in frontal and temporal regions[78][79] and somewhat less widespread cortical thinning in occipital and parietal regions[79] in patients with schizophrenia, relative to healthy control subjects. Moreover, one study decomposed cortical volume into its constituent parts, cortical surface area and cortical thickness, and reported widespread cortical volume reduction in schizophrenia, mainly driven by cortical thinning, but also reduced cortical surface area in smaller frontal, temporal, parietal and occipital cortical regions.[80]
The largest combined neuroimaging study with over 2000 patients and 2500 controls has replicated these previous findings.[81] Here, the authors found volume increases in the lateral ventricles (+18%), caudate and pallidum, and extensive decreases in the hippocampus (-4%), thalamus, amygdala and nucleus accumbens. Together, this indicates that extensive changes occur in vivo in brains in patients suffering from schizophrenia.
DTI
Diffusion tensor imaging (DTI) allows for the investigation of white matter more closely than traditional MRI.[75] Over 300 DTI imaging studies have been published examining white matter abnormalities in schizophrenia.[82][83] Although quite some variation has been found pertaining to the specific regions affected, the general consensus states a reduced fractional anisotropy in brains from patients with schizophrenia versus controls. Importantly, these differences between patients and controls could potentially be attributed to lifestyle effects, medication effects etc. Therefore, more recently several studies have been perform in first-onset schizophrenia patients that have never recent any medication, so-called medication-naive subjects. These studies, although still few in number, also found reduced fractional anisotropy in patient brains compared to control brains. As with earlier findings, abnormalities can be found throughout the brain, although the corpus callous seemed to be most commonly effected. Together, this indicates that abnormalities in white matter could potentially be at the core of the schizophrenia pathophysiology.
CT
Computed Tomography scans of schizophrenic brains show several pathologies. The brain ventricles are enlarged as compared to normal brains. The ventricles hold cerebrospinal fluid (CSF) and enlarged ventricles indicate a loss of brain volume. Additionally, schizophrenic brains have widened sulci as compared to normal brains, also with increased CSF volumes and reduced brain volume.[75][84]
Functional abnormalities
PET
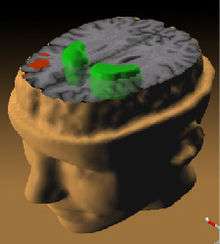
PET scanning is a useful tool to allow the imaging of brain physiology.[86] PET is useful to elaborate hypothesis of the origins of brain pathology, to relate symptoms to biological variables and to study individuals at increased risk. Studies measuring cerebral metabolic rate for glucose (CMRglc) and cerebral blood flow (CBF) have indicated an indirect measurement of synaptic activity. The ability to detect dysfunction of the communication between glutamatergic neurons and astrocytes may lead to an increased understanding of altered functional brain images.[87]
PET scan findings indicate cerebral blood flow decreases in the left parahippocampal region. PET scans also show a reduced ability to metabolize glucose in the thalamus and frontal cortex. PET scans also show involvement of the medial part of the left temporal lobe and the limbic and frontal systems as suffering from developmental abnormality. PET scans show thought disorders stem from increased flow in the frontal and temporal regions while delusions and hallucinations were associated with reduced flow in the cingulate, left frontal, and temporal areas. PET scans done on patient who were actively having auditory hallucinations revealed increased blood flow in both thalami, left hippocampus, right striatum, parahippocampus, orbitofrontal, and cingulate areas.[75]
References
- ↑ van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–45. doi:10.1016/S0140-6736(09)60995-8. PMID 19700006.
- 1 2 Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. doi:10.1192/bjp.188.6.510. PMID 16738340.
- ↑ Insel, T. R. (2010). "Rethinking schizophrenia". Nature. 468 (7321): 187–193. Bibcode:2010Natur.468..187I. doi:10.1038/nature09552. PMID 21068826.
- ↑ Elert, E (2014). "Aetiology: Searching for schizophrenia's roots". Nature. 508 (7494): S2–3. doi:10.1038/508S2a. PMID 24695332.
- ↑ Van Os, J.; Kapur, S. (2009). "Schizophrenia". The Lancet. 374 (9690): 635–645. doi:10.1016/S0140-6736(09)60995-8. PMID 19700006.
- 1 2 Marín, O (2012). "Interneuron dysfunction in psychiatric disorders". Nature Reviews Neuroscience. 13 (2): 107–20. doi:10.1038/nrn3155. PMID 22251963.
- 1 2 Gonzalez-Burgos, G; Cho, R. Y.; Lewis, D. A. (2015). "Alterations in Cortical Network Oscillations and Parvalbumin Neurons in Schizophrenia". Biological Psychiatry. 77 (12): 1031–40. doi:10.1016/j.biopsych.2015.03.010. PMID 25863358.
- 1 2 Pittman-Polletta, B. R.; Kocsis, B; Vijayan, S; Whittington, M. A.; Kopell, N. J. (2015). "Brain Rhythms Connect Impaired Inhibition to Altered Cognition in Schizophrenia". Biological Psychiatry. 77 (12): 1020–30. doi:10.1016/j.biopsych.2015.02.005. PMID 25850619.
- ↑ Feigenson, K. A.; Kusnecov, A. W.; Silverstein, S. M. (2014). "Inflammation and the two-hit hypothesis of schizophrenia". Neuroscience & Biobehavioral Reviews. 38: 72–93. doi:10.1016/j.neubiorev.2013.11.006. PMC 3896922
 . PMID 24247023.
. PMID 24247023. - 1 2 Steullet, P; Cabungcal, J. H.; Monin, A; Dwir, D; O'Donnell, P; Cuenod, M; Do, K. Q. (2014). "Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A "central hub" in schizophrenia pathophysiology?". Schizophrenia Research. doi:10.1016/j.schres.2014.06.021. PMC 4282982
 . PMID 25000913.
. PMID 25000913. - 1 2 Leza, J. C.; García-Bueno, B; Bioque, M; Arango, C; Parellada, M; Do, K; O'Donnell, P; Bernardo, M (2015). "Inflammation in schizophrenia: A question of balance". Neuroscience & Biobehavioral Reviews. 55: 612–26. doi:10.1016/j.neubiorev.2015.05.014. PMID 26092265.
- ↑ Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U.S.A. 1996;93(17):9235–40. doi:10.1073/pnas.93.17.9235. PMID 8799184.
- ↑ Jones HM, Pilowsky LS. Dopamine and antipsychotic drug action revisited. Br J Psychiatry. 2002;181:271–5. doi:10.1192/bjp.181.4.271. PMID 12356650.
- ↑ Martin S. An Atlas of Schizophrenia. Washington, DC: Taylor & Francis; 2002. ISBN 1-85070-074-5. p. 54.
- ↑ Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–3. doi:10.1126/science.3854. PMID 3854.
- ↑ Angrist B, van Kammen DP. CNS stimulants as a tool in the study of schizophrenia. Trends in Neurosciences. 1984;7:388–90. doi:10.1016/S0166-2236(84)80062-4.
- ↑ Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl.). 1987;91(4):415–33. doi:10.1007/BF00216006. PMID 2884687.
- ↑ Myers DG. Schizophrenia. Psychology. 2007:678–85.
- ↑ Davis C, Jeste D, Eyler L. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophrenia Research. 1 October 2005;78(1):45–60. doi:10.1016/j.schres.2005.05.009. PMID 15979287.
- ↑ Gur RE, Chin S. Laterality in functional brain imaging studies of schizophrenia.. Schizophrenia bulletin. 1999;25(1):141–56. doi:10.1093/oxfordjournals.schbul.a033361. PMID 10098918.
- ↑ Meisenzahl EM, Schmitt GJ, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. International Review of Psychiatry. 2007;19(4):337–45. doi:10.1080/09540260701502468. PMID 17671867.
- ↑ Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42(3):211–21. PMID 9796369.
- ↑ Stone JM, Morrison PD, Pilowsky LS. Review: Glutamate and dopamine dysregulation in schizophrenia a synthesis and selective review. Journal of Psychopharmacology. 2006;21(4):440–52. doi:10.1177/0269881106073126. PMID 17259207.
- ↑ Maas JW, Contreras SA, Seleshi E, Bowden CL. Dopamine metabolism and disposition in schizophrenic patients. Studies using debrisoquin. Arch Gen Psychiatry. 1988;45(6):553–9. doi:10.1001/archpsyc.1988.01800300049005. PMID 3377641.
- 1 2 Seeman P, Schwarz J, Chen JF, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60(4):319–46. doi:10.1002/syn.20303. PMID 16786561.
- ↑ Safra, JE (Chairman) 2005 'Cooperativity' The New Encyclopaedia Britannica, Vol 3, Micropaedia, p 666
- ↑ Fuxe K, Marcellino D, Guidolin D, Woods A, Agnati L, Chapter 10 – Dopamine Receptor Oligermization, in Neve KA (ed)'Dopamine Receptors' Springer (2009)
- 1 2 "Dopamine Receptors: Clinical Correlates". Acnp.org. Retrieved 2015-05-26.
- ↑ Seeman, P.; Guan, H. C.; Civelli, O.; Van Tol, H. H. M.; Sunahara, R. K.; Niznik, H. B. (1992). "The cloned dopamine D2 receptor reveals different densities for dopamine receptor antagonist ligands. Implications for human brain positron emission tomography". European Journal of Pharmacology: Molecular Pharmacology. 227 (2): 139–146. doi:10.1016/0922-4106(92)90121-B.
- ↑ Zakzanis KK, Hansen KT. Dopamine D2 densities and the schizophrenic brain. Schizophrenia Research. 1998;32(3):201–6. doi:10.1016/s0920-9964(98)00041-3. PMID 9720125.
- ↑ Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97(14):7673–5. doi:10.1073/pnas.97.14.7673. PMID 10884398.
- ↑ Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U.S.A.. 2000;97(14):8104–9. doi:10.1073/pnas.97.14.8104. PMID 10884434.
- ↑ Vernaleken I, Eickhoff S, Veselinovic T, et al. Elevated D2/3-receptor availability in schizophrenia: A [18F]fallypride study. NeuroImage. 2008;41:T145. doi:10.1016/j.neuroimage.2008.04.113.
- ↑ Cropley VL, Innis RB, Nathan PJ, et al. Small effect of dopamine release and no effect of dopamine depletion on 18Ffallypride binding in healthy humans.. Synapse. 2008;62(6):399–408. doi:10.1002/syn.20506. PMID 18361438.
- ↑ Abi-Dargham A, Elsmarieke E, Slifstein M, Kegeles LS, Laruelle M. Baseline and Amphetamine-Stimulated Dopamine Activity Are Related in Drug-Naïve Schizophrenic Subjects. Biological Psychiatry. 2009;65(12):1091–3. doi:10.1016/j.biopsych.2008.12.007. PMID 19167701.
- ↑ "Amphetamine psychosis has been proposed as a model for some features of schizophrenia... This model of amphetamine sensitization has also been adopted as a paradigm for researchers interested in the addictive powers of drugs of abuse."
- ↑ Bradley KC, Meisel RL. Sexual Behavior Induction of c-Fos in the Nucleus Accumbens and Amphetamine-Stimulated Locomotor Activity Are Sensitized by Previous Sexual Experience in Female Syrian Hamsters. J. Neurosci. 2001;21(6):2123–30. PMID 11245696.
- ↑ Wong DF, Wagner H, Tune L, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234(4783):1558–63. doi:10.1126/science.2878495. PMID 2878495.
- ↑ Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404–16. doi:10.1177/1073858403252674. PMID 14580124.
- ↑ Seeman P, Guan H-C, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365(6445):441. doi:10.1038/365441a0. PMID 8413587.
- ↑ For a discussion of opposing studies see p 143: Seeman P, Guan H-C, Nobrega J et al. Synapse. 1997;25(2):137–46. doi:10.1002/(SICI)1098-2396(199702)25:2<137::AID-SYN4>3.0.CO;2-D. PMID 9021894.
- ↑ Levitzki A, Schlessinger J. Cooperativity in associating proteins. Monomer-dimer equilibrium coupled to ligand binding. Biochemistry. 1974;13(25):5214–9. doi:10.1021/bi00722a026. PMID 4433518.
- ↑ Seeman P. All Psychotic Roads Lead to Increased Dopamine D2High Receptors: A Perspective. Clinical Schizophrenia & Related Psychoses. 2008;1:351–5. doi:10.3371/CSRP.1.4.7.
- ↑ Seeman P, Van Tol HH. Dopamine receptor pharmacology.. Trends in pharmacological sciences. 1994;15(7):264–70. doi:10.1016/0165-6147(94)90323-9. PMID 7940991.
- ↑ Schizophrenia Working Group of the Psychiatric Genomics Consortium; Neale, Benjamin M.; Corvin, Aiden; Walters, James T. R.; Farh, Kai-How; Holmans, Peter A.; Lee, Phil; Bulik-Sullivan, Brendan; Collier, David A.; Huang, Hailiang; Pers, Tune H.; Agartz, Ingrid; Agerbo, Esben; Albus, Margot; Alexander, Madeline; Amin, Farooq; Bacanu, Silviu A.; Begemann, Martin; Belliveau Jr, Richard A.; Bene, Judit; Bergen, Sarah E.; Bevilacqua, Elizabeth; Bigdeli, Tim B.; Black, Donald W.; Bruggeman, Richard; Buccola, Nancy G.; Buckner, Randy L.; Byerley, William; Cahn, Wiepke; et al. (2014). "Biological insights from 108 schizophrenia-associated genetic loci". Nature. 511 (7510): 421–7. doi:10.1038/nature13595. PMC 4112379
 . PMID 25056061.
. PMID 25056061. - ↑ Chun, S; Westmoreland, J. J.; Bayazitov, I. T.; Eddins, D; Pani, A. K.; Smeyne, R. J.; Yu, J; Blundon, J. A.; Zakharenko, S. S. (2014). "Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models". Science. 344 (6188): 1178–82. doi:10.1126/science.1253895. PMC 4349506
 . PMID 24904170.
. PMID 24904170. - ↑ Kapur, S (2003). "Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia". The American Journal of Psychiatry. 160 (1): 13–23. doi:10.1176/appi.ajp.160.1.13. PMID 12505794.
- ↑ Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther. 2003;97(2):153–79. doi:10.1016/S0163-7258(02)00328-5. PMID 12559388.
- ↑ Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–67. doi:10.1016/S0893-133X(01)00243-3. PMID 11557159.
- ↑ Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Annals of the New York Academy of Sciences. 2003;1003:318–27. doi:10.1196/annals.1300.020. PMID 14684455.
- ↑ Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2005;72(2-3):225–34. doi:10.1016/j.schres.2004.05.005. PMID 15560967.
- ↑ Agronin, ME; Maletta GJ (2006). Principles And Practice Of Geriatric Psychiatry. Lippincott Williams & Wilkins. p. 215. ISBN 0-7817-4810-0.
- ↑ Weickert, C. S.; Fung, S. J.; Catts, V. S.; Schofield, P. R.; Allen, K. M.; Moore, L. T.; Newell, K. A.; Pellen, D; Huang, X. F.; Catts, S. V.; Weickert, T. W. (2013). "Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia". Molecular Psychiatry. 18 (11): 1185–92. doi:10.1038/mp.2012.137. PMC 3807670
 . PMID 23070074.
. PMID 23070074. - ↑ The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view.. J Neural Transm Suppl. 2007;(72):269–80. PMID 17982903.
- 1 2 Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders.. CNS Drugs. 2009;23(2):91–101. doi:10.2165/00023210-200923020-00001. PMID 19173370.
- ↑ Endogenous neuro-protectants in ammonia toxicity in the central nervous system: facts and hypotheses.. Metab Brain Dis. Dec 2005;20(4):253–63. doi:10.1007/s11011-005-7904-6. PMID 16382336.
- ↑ The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression.. Mol Psychiatry. Mar 2014;19(3):334–41. doi:10.1038/mp.2013.11. PMID 23459468.
- ↑ Possible impact of microglial cells and the monocyte-macrophage system on suicidal behavior.. CNS Neurol Disord Drug Targets. Nov 2013;12(7):971–9. doi:10.2174/18715273113129990099. PMID 24040799.
- ↑ Gonzalez-Burgos, G; Hashimoto, T; Lewis, D. A. (2010). "Alterations of cortical GABA neurons and network oscillations in schizophrenia". Current Psychiatry Reports. 12 (4): 335–44. doi:10.1007/s11920-010-0124-8. PMC 2919752
 . PMID 20556669.
. PMID 20556669. - ↑ Konradi, C; Yang, C. K.; Zimmerman, E. I.; Lohmann, K. M.; Gresch, P; Pantazopoulos, H; Berretta, S; Heckers, S (2011). "Hippocampal interneurons are abnormal in schizophrenia". Schizophrenia Research. 131 (1–3): 165–73. doi:10.1016/j.schres.2011.06.007. PMC 3159834
 . PMID 21745723.
. PMID 21745723. - ↑ Lu CL, Wang YC, Chen JY, Lai IC, Liou YJ. Support for the involvement of the ERBB4 gene in schizophrenia: a genetic association analysis. Neurosci. Lett. 2010;481(2):120–5. doi:10.1016/j.neulet.2010.06.067. PMID 20600594.
- ↑ Pocklington, A. J.; Rees, E; Walters, J. T.; Han, J; Kavanagh, D. H.; Chambert, K. D.; Holmans, P; Moran, J. L.; McCarroll, S. A.; Kirov, G; O'Donovan, M. C.; Owen, M. J. (2015). "Novel Findings from CNVs Implicate Inhibitory and Excitatory Signaling Complexes in Schizophrenia". Neuron. 86 (5): 1203–14. doi:10.1016/j.neuron.2015.04.022. PMC 4460187
 . PMID 26050040.
. PMID 26050040. - ↑ Kinney DK, Hintz K, Shearer EM, Barch DH, Riffin C, Whitley K, Butler R. A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med. Hypotheses. 2010;74(3):555–63. doi:10.1016/j.mehy.2009.09.040. PMID 19836903.
- ↑ Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, Agartz I, Ueland T, Andreassen OA. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. Nov 2009;11(7):726–34. doi:10.1111/j.1399-5618.2009.00757.x. PMID 19839997.
- ↑ Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen Lv, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10(1):59–76. doi:10.1586/ern.09.144. PMID 20021321.
- ↑ Hope, S; Ueland, T; Steen, N. E.; Dieset, I; Lorentzen, S; Berg, A. O.; Agartz, I; Aukrust, P; Andreassen, O. A. (2013). "Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder". Schizophrenia Research. 145 (1–3): 36–42. doi:10.1016/j.schres.2012.12.023. PMID 23403415.
- ↑ Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium; Sanders, Alan R; Kendler, Kenneth S; Levinson, Douglas F; Sklar, Pamela; Holmans, Peter A; Lin, Dan-Yu; Duan, Jubao; Ophoff, Roel A; Andreassen, Ole A; Scolnick, Edward; Cichon, Sven; St. Clair, David; Corvin, Aiden; Gurling, Hugh; Werge, Thomas; Rujescu, Dan; Blackwood, Douglas H R; Pato, Carlos N; Malhotra, Anil K; Purcell, Shaun; Dudbridge, Frank; Neale, Benjamin M; Rossin, Lizzy; Visscher, Peter M; Posthuma, Danielle; Ruderfer, Douglas M; Fanous, Ayman; Stefansson, Hreinn; et al. (2011). "Genome-wide association study identifies five new schizophrenia loci". Nature Genetics. 43 (10): 969–76. doi:10.1038/ng.940. PMC 3303194
 . PMID 21926974.
. PMID 21926974. - ↑ Emiliani, F. E.; Sedlak, T. W.; Sawa, A (2014). "Oxidative stress and schizophrenia: Recent breakthroughs from an old story". Current Opinion in Psychiatry. 27 (3): 185–90. doi:10.1097/YCO.0000000000000054. PMC 4054867
 . PMID 24613987.
. PMID 24613987. - ↑ Monin, A; Baumann, P. S.; Griffa, A; Xin, L; Mekle, R; Fournier, M; Butticaz, C; Klaey, M; Cabungcal, J. H.; Steullet, P; Ferrari, C; Cuenod, M; Gruetter, R; Thiran, J. P.; Hagmann, P; Conus, P; Do, K. Q. (2015). "Glutathione deficit impairs myelin maturation: Relevance for white matter integrity in schizophrenia patients". Molecular Psychiatry. 20 (7): 827–38. doi:10.1038/mp.2014.88. PMID 25155877.
- ↑ Cabungcal, J. H.; Steullet, P; Kraftsik, R; Cuenod, M; Do, K. Q. (2013). "Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by N-acetylcysteine". Biological Psychiatry. 73 (6): 574–82. doi:10.1016/j.biopsych.2012.09.020. PMID 23140664.
- ↑ Flashman LA, Green MF. Review of cognition and brain structure in schizophrenia: profiles, longitudinal course, and effects of treatment. Psychiatr. Clin. North Am. 2004;27(1):1–18, vii. doi:10.1016/S0193-953X(03)00105-9. PMID 15062627.
- ↑ Lieberman JA, Bymaster FP, Meltzer HY, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 2008;60(3):358–403. doi:10.1124/pr.107.00107. PMID 18922967.
- ↑ DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34(2):312–21. doi:10.1093/schbul/sbm164. PMID 18263882.
- 1 2 Jung WH, Jang JH, Byun MS, An SK, Kwon JS. Structural Brain Alterations in Individuals at Ultra-high Risk for Psychosis: A Review of Magnetic Resonance Imaging Studies and Future Directions. J. Korean Med. Sci. 2010;25(12):1700–9. doi:10.3346/jkms.2010.25.12.1700. PMID 21165282.
- 1 2 3 4 Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49(1-2):1–52. doi:10.1016/s0920-9964(01)00163-3. PMID 11343862.
- ↑ Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–45. doi:10.1176/appi.ajp.162.12.2233. PMID 16330585.
- ↑ Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr. Res. 2006;82(1):75–88. doi:10.1016/j.schres.2005.11.004. PMID 16377156.
- ↑ Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–88. doi:10.1001/archpsyc.60.9.878. PMID 12963669.
- 1 2 Rimol, L.M., Hartberg, C.B., Nesvag, R., Fennema-Notestine, C., Hagler, D.J., Jr., Pung, C.J., Jennings, R.G., Haukvik, U.K., Lange, E., Nakstad, P.H., Melle, I., Andreassen, O.A., Dale, A.M., Agartz, I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol.Psychiatry. 2010;68(1):41–50. doi:10.1016/j.biopsych.2010.03.036. PMID 20609836.
- ↑ Rimol L.M., Nesvåg R., Hagler D. Jr, Bergmann Ø., Fennema-Notestine C., Hartberg C.B., Haukvik U.K., Lange E., Pung C.J., Server A., Melle I., Andreassen O.A., Agartz I., Dale A.M. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol.Psychiatry. 2012;71(6):552–60. doi:10.1016/j.biopsych.2011.11.026. PMID 22281121.
- ↑ Van Erp, T. G.; Hibar, D. P.; Rasmussen, J. M.; Glahn, D. C.; Pearlson, G. D.; Andreassen, O. A.; Agartz, I; Westlye, L. T.; Haukvik, U. K.; Dale, A. M.; Melle, I; Hartberg, C. B.; Gruber, O; Kraemer, B; Zilles, D; Donohoe, G; Kelly, S; McDonald, C; Morris, D. W.; Cannon, D. M.; Corvin, A; Machielsen, M. W.; Koenders, L; De Haan, L; Veltman, D. J.; Satterthwaite, T. D.; Wolf, D. H.; Gur, R. C.; Gur, R. E.; et al. (2015). "Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium". Molecular Psychiatry. doi:10.1038/mp.2015.63. PMC 4668237
 . PMID 26033243.
. PMID 26033243. - ↑ Fitzsimmons, J; Kubicki, M; Shenton, M. E. (2013). "Review of functional and anatomical brain connectivity findings in schizophrenia". Current Opinion in Psychiatry. 26 (2): 172–87. doi:10.1097/YCO.0b013e32835d9e6a. PMID 23324948.
- ↑ Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108(1-3):3–10. doi:10.1016/j.schres.2008.11.021. PMID 19128945.
- ↑ Ropper AH, Brown RH. Adams and Victor's Principles of Neurology. 8th ed. New York: McGraw-Hill; 2005 [Retrieved 2010-12-28]. ISBN 0-07-141620-X. p. 1324.
- ↑ Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5(3):267–71. doi:10.1038/nn804. PMID 11865311.
- ↑ Vyas NS, Patel NH, Nijran KS, Al-Nahhas A, Puri BK. The use of PET imaging in studying cognition, genetics and pharmacotherapeutic interventions in schizophrenia. Expert Rev Neurother. 2011;11(1):37–51. doi:10.1586/ern.10.160. PMID 21158554.
- ↑ Giovacchini G, Ferdinando S, Esmaeilzadeh M, Amalia M, Luigi M, Ciarmiello A. Pet translates neurophysiology in images : A review to stimulate a network between neuroimaging and basic research. J. Cell. Physiol. 2010. doi:10.1002/jcp.22451. PMID 20945377.