Heck reaction
| Heck reaction | |
|---|---|
| Named after | Richard F. Heck |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | heck-reaction |
| RSC ontology ID | RXNO:0000024 |
The Heck reaction (also called the Mizoroki-Heck reaction)[1] is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene.[2][3] It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is of great importance, as it allows one to do substitution reactions on planar sp2-hybridized carbon atoms.
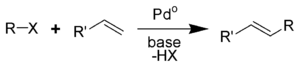 |
| The Heck reaction |
|---|
The reaction is performed in the presence of an organopalladium catalyst. The halide (Br, Cl)[4] or triflate is an aryl, benzyl, or vinyl compound and the alkene contains at least one hydrogen and is often electron-deficient such as acrylate ester or an acrylonitrile.The catalyst can be tetrakis(triphenylphosphine)palladium(0), palladium chloride or palladium(II) acetate. The ligand is triphenylphosphine, PHOX or BINAP. The base is triethylamine, potassium carbonate or sodium acetate.
Several reviews have been published.[5][6][7][8]
History
The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene to form stilbene in methanol at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was an extension of earlier work by Fujiwara (1967) on the Pd(II)-mediated coupling of arenes (Ar–H) and alkenes[9][10] and earlier work by Heck (1969) on the coupling of arylmercuric halides (ArHgCl) with alkenes using a stoichiometric amount of a palladium(II) species.[11]
 |
| Mizoroki 1971 |
|---|
The 1972 Heck publication acknowledged the Mizoroki publication and detailed independently discovered work. The reaction conditions differ in catalyst used (palladium acetate) and catalyst loading (0.01 eq.), base used (a hindered amine) and lack of solvent.
 |
| Heck 1972 |
|---|
In these reactions the active catalyst Pd(0) (see reaction mechanism) is formed by Pd coordination to the alkene.
In 1974 Heck introduced phosphine ligands into the equation.[12]
 |
| Heck reaction 1974 phosphines |
|---|
Reaction mechanism
The catalytic cycle for the Heck reaction involves a series of transformations around the palladium catalyst. The palladium(0) compound required in this cycle is generally prepared in situ from a palladium(II) precursor.[13][14]
For instance, palladium(II) acetate is reduced by triphenylphosphine to bis(triphenylphosphine)palladium(0) (1) and triphenylphosphine is oxidized to triphenylphosphine oxide. Step A is an oxidative addition in which palladium inserts itself in the aryl to bromide bond. Palladium then forms a π complex with the alkene (3) and in step B the alkene inserts itself in the palladium - carbon bond in a syn addition step. Then follows a torsional strain relieving rotation to the trans isomer (not shown) and step C is a beta-hydride elimination step with the formation of a new palladium - alkene π complex (5). This complex is destroyed in the next step. The palladium(0) compound is regenerated by reductive elimination of the palladium(II) compound by potassium carbonate in the final step, D. In the course of the reaction the carbonate is stoichiometrically consumed and palladium is truly a catalyst and used in catalytic amounts. A similar palladium cycle but with different scenes and actors is observed in the Wacker process.
 |
| Heck Reaction Mechanism |
|---|
This cycle is not limited to vinyl compounds, in the Sonogashira coupling one of the reactants is an alkyne and in the Suzuki coupling the alkene is replaced by an aryl boronic acid and in the Stille reaction by an aryl stannane. The cycle also extends to the other group 10 element nickel for example in the Negishi coupling between aryl halides and organozinc compounds. Platinum forms strong bonds with carbon and does not have a catalytic activity in this type of reaction.
Stereoselectivity
This coupling reaction is stereoselective with a propensity for trans coupling as the palladium halide group and the bulky organic residue move away from each other in the reaction sequence in a rotation step. The Heck reaction is applied industrially in the production of naproxen and the sunscreen component octyl methoxycinnamate. The naproxen synthesis includes a coupling between a brominated naphthalene compound with ethylene:[15]
 |
| The Heck reaction in Naproxen production |
|---|
Variations
Ionic liquid Heck reaction
In the presence of an ionic liquid a Heck reaction proceeds in absence of a phosphorus ligand. In one modification palladium acetate and the ionic liquid (bmim)PF6 are immobilized inside the cavities of reversed-phase silica gel.[16] In this way the reaction proceeds in water and the catalyst is re-usable.
 |
| Siloxane application |
|---|
Heck oxyarylation
In the Heck oxyarylation modification the palladium substituent in the syn-addition intermediate is displaced by a hydroxyl group and the reaction product contains a dihydrofuran ring.[17]
 |
| Heck oxyarylation |
|---|
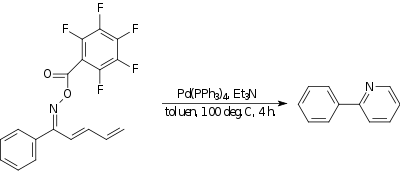
Amino-Heck reaction
In the amino-Heck reaction a nitrogen to carbon bond is formed. In one example,[18] an oxime with a strongly electron withdrawing group reacts intramolecularly with the terminal end of a diene to a pyridine compound. The catalyst is tetrakis(triphenylphosphine)palladium(0) and the base is triethylamine.
 |
| Amino-Heck reaction |
|---|
See also
References
- ↑ Drahl, Carmen (May 17, 2010). "In Names, History And Legacy". Chemical and Engineering News. 88 (22): 31–33. doi:10.1021/cen-v088n020.p031. Retrieved June 4, 2011.
- ↑ Heck, R. F.; Nolley, Jr., J. P. (1972). "Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides". J. Org. Chem. 37 (14): 2320–2322. doi:10.1021/jo00979a024.
- ↑ Mizoroki, T.; Mori, K.; Ozaki, A. (1971). "Arylation of Olefin with Aryl Iodide Catalyzed by Palladium". Bull. Chem. Soc. Jap. 44 (2): 581. doi:10.1246/bcsj.44.581.
- ↑ Littke, A. F.; Fu, G. C. (2005). "Heck reactions of aryl chlorides catalyzed by palladium/tri-tert-butylphosphine: (E)-2-Methyl-3-phenylacrylic acid butyl ester and (E)-4-(2-phenylethenyl)benzonitrile". Org. Synth. 81: 63.
- ↑ Heck, R. F. (1982). "Palladium-catalyzed vinylation of organic halides". Org. React. 27: 345–390. doi:10.1002/0471264180.or027.02.
- ↑ A. de Meijere, F. E. Meyer, Jr.; (1994). "Fine Feathers Make Fine Birds: The Heck Reaction in Modern Garb". Angew. Chem. Int. Ed. Engl. 33 (2324): 2379–2411. doi:10.1002/anie.199423791.
- ↑ Beletskaya, I. P.; Cheprakov, A. V. (2000). "The Heck Reaction as a Sharpening Stone of Palladium Catalysis". Chem. Rev. 100 (8): 3009–3066. doi:10.1021/cr9903048. PMID 11749313.
- ↑ Mc Cartney, Dennis; Guiry, Patrick J. (2011). "The asymmetric Heck and related reactions". Chemical Society Reviews. 40 (10): 5122–5150. doi:10.1039/C1CS15101K. PMID 21677934.
- ↑ Moritani, Ichiro; Fujiwara, Yuzo (1967). "Aromatic substitution of styrene-palladium chloride complex". Tetrahedron Letters. 8 (12): 1119–1122. doi:10.1016/S0040-4039(00)90648-8.
- ↑ Fujiwara, Yuzo; Noritani, Ichiro; Danno, Sadao; Asano, Ryuzo; Teranishi, Shiichiro (1969). "Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate". Journal of the American Chemical Society. 91 (25): 7166. doi:10.1021/ja01053a047.
- ↑ Heck, Richard F. (1969). "Mechanism of arylation and carbomethoxylation of olefins with organopalladium compounds". Journal of the American Chemical Society. 91 (24): 6707. doi:10.1021/ja01052a029.
- ↑ Dieck, H. A.; Heck, R. F. (1974). "Organophosphinepalladium complexes as catalysts for vinylic hydrogen substitution reactions". Journal of the American Chemical Society. 96 (4): 1133. doi:10.1021/ja00811a029.
- ↑ Ozawa, F.; Kubo, A.; Hayashi, T. (1992). "Generation of Tertiary Phosphine-Coordinated Pd(0) Species from Pd(OAc)2 in the Catalytic Heck Reaction". Chemistry Lett. (11): 2177–2180. doi:10.1246/cl.1992.2177.
- ↑ Bradshaw, Michael; Zou, Jianli; Byrne, Lindsay; Swaminathan Iyer, K.; Stewart, Scott G.; Raston, Colin L. (October 6, 2011). "Pd(II) conjugated chitosan nanofibre mats for application in Heck cross-coupling reactions". Chemical Communications. 47 (45): 12292–12294. doi:10.1039/C1CC14717J. PMID 22011792. Retrieved October 20, 2011.
- ↑ De Vries; Johannes G. (2001). "The Heck reaction in the production of fine chemicals". Canadian Journal of Chemistry. 79 (5–6): 1086. doi:10.1139/cjc-79-5-6-1086.
- ↑ Hagiwara, Hisahiro; Sugawara, Yoshitaka; Hoshi, Takashi; Suzuki, Toshio (2005). "Sustainable Mizoroki–Heck reaction in water: remarkably high activity of Pd(OAc)2 immobilized on reversed phase silica gel with the aid of an ionic liquid". Chemical Communications (23): 2942–2944. doi:10.1039/b502528a. PMID 15957033.
- ↑ Lorand Kiss; Tibor Kurtan; Sandor Antus; Henri Brunner (2003). "Further insight into the mechanism of Heck oxyarylation in the presence of chiral ligands". Arkivoc: GB–653J.
- ↑ Mitsuru Kitamura; Daisuke Kudo; Koichi Narasaka (2005). "Palladium(0)-catalyzed synthesis of pyridines from β-acetoxy-γ,δ-unsaturated ketone oximes". Arkivoc: JC–1563E.
External links
| Wikimedia Commons has media related to Heck reaction. |
- The Heck reaction at organic-chemistry.org Article
- Heck reaction: synthetic protocols from organic-reaction.com