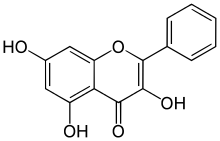
Galangin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,5,7-trihydroxy-2-phenylchromen-4-one | |
| Other names
Norizalpinin 3,5,7-Trihydroxyflavone 3,5,7-triOH-Flavone | |
| Identifiers | |
| 548-83-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:5262 |
| ChEMBL | ChEMBL309490 |
| ChemSpider | 4444935 |
| ECHA InfoCard | 100.008.147 |
| 410 | |
| KEGG | C10044 |
| PubChem | 5281616 |
| UNII | 142FWE6ECS |
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.24 g/mol |
| Density | 1.579 g/mL |
| Melting point | 214 to 215 °C (417 to 419 °F; 487 to 488 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Galangin is a flavonol, a type of flavonoid. It is found in high concentrations in Alpinia officinarum (lesser galangal)[1] and Helichrysum aureonitens.[2] It is also found in the galangal rhizome (Alpinia galanga)[3] and in propolis.[4] Galangin has been shown to have in vitro antibacterial[5][6] and antiviral activity.[7] The flavonol also inhibits the growth of breast tumor cells in vitro.[8][9]
See also
References
- ↑ Ciolino, H. P.; Yeh, G. C. (1999). "The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor". British Journal of Cancer. 79 (9/10): 1340–1346. doi:10.1038/sj.bjc.6690216.
- ↑ Afolayan AJ, Meyer JJ (1997). "The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens". Journal of Ethnopharmacology. 57 (3): 177–181. doi:10.1016/s0378-8741(97)00065-2. PMID 9292410.
- ↑ Kaur, A.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P. (2010). "Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd" (PDF). Indian Journal of Experimental Biology. 48 (3): 314–317. PMID 21046987.
- ↑ Tosi, E; Re, E; Ortega, M; Cazzoli, A (2007). "Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli". Food Chemistry. 104: 1025. doi:10.1016/j.foodchem.2007.01.011.
- ↑ Cushnie TP, Lamb AJ (2006). "Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus". Phytomedicine. 13 (3): 187–191. doi:10.1016/j.phymed.2004.07.003. PMID 16428027.
- ↑ Cushnie TP, Lamb AJ (2005). "Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss". Journal of Ethnopharmacology. 101 (1-3): 243–248. doi:10.1016/j.jep.2005.04.014. PMID 15985350.
- ↑ Afolayan AJ, Meyer JJ, Taylor MB, Erasmus D (1997). "Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens". Journal of Ethnopharmacology. 56 (2): 165–169. doi:10.1016/s0378-8741(97)01514-6. PMID 917497.
- ↑ So, F. V.; Guthrie, N.; Chambers, A. F.; Moussa, M.; Carroll, K. K. (1996). "Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices". Nutrition and Cancer. 26 (2): 167–181. doi:10.1080/01635589609514473. PMID 8875554.
- ↑ So, F.; Guthrie, N.; Chambers, A. F.; Carroll, K. K. (1997). "Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen". Cancer Letters. 112 (2): 127–133. doi:10.1016/S0304-3835(96)04557-0. PMID 9066718.
External links
This article is issued from Wikipedia - version of the 5/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.