Cdc6
| Cdc6 | |
|---|---|
|
Crystal Structure of CDC6p from P. aerophilum.[1] | |
| Identifiers | |
| Symbol | Cdc6 |
| Alt. symbols | YJL194W |
| Entrez | 853244 |
| PDB | 1FNN |
| UniProt | P09119 |
Cdc6, or cell division cycle 6, is a protein in eukaryotic cells that is studied in the budding yeast Saccharomyces cerevisiae. It is an essential regulator of DNA replication and plays important roles in the activation and maintenance of the checkpoint mechanisms in the cell cycle that coordinate S phase and mitosis. It is part of the pre-replicative complex (pre-RC) and is required for loading mini chromosome maintenance (MCM) proteins onto the DNA, an essential step in the initiation of DNA synthesis. In addition, it is a member of the family of AAA+ ATPases and highly associated to Orc1p.
Function

Cdc6p is an ATP binding protein and a member of the pre-replicative complex (pre-RC) together with the origin recognition complex (ORC), Cdt1 and the MCM complex (containing MCM2-7p). Cdc6p assembles after ORC in an ATP dependent manner and is required for loading MCM proteins onto the DNA. Reconstruction of electron microscope images showed that the ORC-Cdc6p complex forms a ring-shaped structure with similar dimensions to those of ring-shaped MCM helicase.[3] It is thought that the Cdc6p-Cdt1 complex uses ATP hydrolysis to thread DNA through the central hole of the MCM doughnut.[4] Mutations in the binding motif of Cdc6p strongly suggest that ATP binding and hydrolysis is essential for its function.[5] The minimal requirement for DNA binding has been mapped within its 47-amino acid sequence.[6] Furthermore, Cdc6 indirectly inhibits activation of the p34cdc2/CDC28 M phase kinase, thus nuclear division is suppressed.[7]
Regulation
Cdc6p is normally present at high levels during the G1 phase of the cell cycle. This is partly because the CDC6 gene is only transcribed during G1 phase. On the onset of the S phase, Cdc6p gets phosphorylated by the Cdc28-Clb5-Clb6 complex (Cdk1) and consequently becoming inactivated. This has been shown by introducing mutations in Cdc6p at the consensus sites for Cdk1 phosphorylation (near the N-terminus) which inhibit degradation. The phosphorylation can furthermore be catalyzed by Cdc28-Cln. The inactivated Cdc6p is then targeted for degradation by SCFCDC4-dependent ubiquitinylation and afterwards degraded by the proteasome. Thus, the regulation of Cdc6p is tightly correlated to the activity of Cdk1 and since Cdk1-activity is oscillating once per cell cycle, the accumulation and degradation of Cdc6p also oscillates.
Two states can be distinguished. In the first state (during G1 phase) Cdk1-activity is low, Cdc6p can accumulate, hence the pre-RC can be formed but not activated. In the second state Cdk1-activity is high, Cdc6p becomes inactivated, hence the pre-RC is activated but not formed. This change assures that DNA replication is performed only once per cell cycle. It has been shown that overexpression of Cdc6p does not induce re-replication in cognate cells, probably due to inhibition through CDK that resets the cell cycle clock to G1. Nevertheless, it has been suggested that regulation of Cdc6p is one of several redundant mechanisms that prevent re-replication of the DNA in eukaryotic cells.[8]
Structure
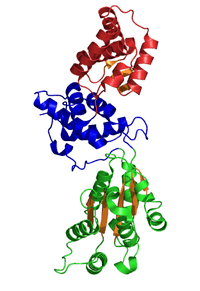
The crystallographic structure of a Cdc6p/Orc1-related protein from Pyrobaculum aerophilum (see Pyrobaculum) has been solved and three structural domains have been identified. Domain I and II form the ATP binding/hydrolysis site and are similar to other AAA+ ATPases. Domain III is structurally related to a winged-helix domain, thus may interact with origin DNA. From studies with E. coli γ clamp loading complex, it was suggested that domain III mediates protein-protein interactions with other AAA+ ATPases in the pre-RC, thus suggesting that the Cdc6p builds a homodimer in its native form. The domains I and II form a cashew-shaped molecule that bind ATP in the cleft and additionally build the sensor motif for ATP/ADP recognition. These domains are also thought to mediate subsequent conformational changes. Nevertheless, the exact functional roles of these domains remain unclear.[6]
Disease
It has been shown Cdc6p shows proto-oncogenic activity. Cdc6 overexpression interferes with the expression of INK4/ARF tumor suppressor genes through a mechanism involving the epigenetic modification of chromatin at the INK4/ARF locus. In addition, Cdc6p overexpression in primary cells may promote DNA hyperreplication and induce a senescence response similar to that caused by oncogene activation. These findings indicate that deregulation of CDC6 expression in human cells poses a serious risk of carcinogenesis.[2] Down-regulation of CDC6 in prostate cancer was observed and associated with phenotypic characteristics of aggressive prostate cancer.[9] Furthermore, it has been observed that Cdc6 is greatly up-regulated in cervical cancer, lung cancer and brain cancer.[10]
See also
References
- ↑ PDB: 1FNNLiu J, Smith CL, DeRyckere D, DeAngelis K, Martin GS, Berger JM (September 2000). "Structure and function of Cdc6/Cdc18: implications for origin recognition and checkpoint control". Mol. Cell. 6 (3): 637–48. doi:10.1016/S1097-2765(00)00062-9. PMID 11030343.
- 1 2 Borlado LR, Méndez J (February 2008). "CDC6: from DNA replication to cell cycle checkpoints and oncogenesis". Carcinogenesis. 29 (2): 237–43. doi:10.1093/carcin/bgm268. PMID 18048387.
- ↑ Speck C, Chen Z, Li H, Stillman B (November 2005). "ATPase-dependent cooperative binding of ORC and Cdc6 to origin DNA". Nat. Struct. Mol. Biol. 12 (11): 965–71. doi:10.1038/nsmb1002. PMC 2952294
 . PMID 16228006.
. PMID 16228006. - ↑ Jennifer Lippincott-Schwartz; Pollard, Thomas D.; Earnshaw, William (2007). Cell biology. Saunders Elsevier. pp. 766–767. ISBN 1-4160-2255-4.
- ↑ Bell SP, Dutta A (2002). "DNA replication in eukaryotic cells". Annu. Rev. Biochem. 71: 333–74. doi:10.1146/annurev.biochem.71.110601.135425. PMID 12045100.
- 1 2 Feng L, Wang B, Driscoll B, Jong A (May 2000). "Identification and characterization of Saccharomyces cerevisiae Cdc6 DNA-binding properties". Mol. Biol. Cell. 11 (5): 1673–85. doi:10.1091/mbc.11.5.1673. PMC 14875
 . PMID 10793143.
. PMID 10793143. - ↑ Bueno A, Russell P (June 1992). "Dual functions of CDC6: a yeast protein required for DNA replication also inhibits nuclear division". EMBO J. 11 (6): 2167–76. PMC 556684
 . PMID 1600944.
. PMID 1600944. - ↑ Drury LS, Perkins G, Diffley JF (October 1997). "The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast". EMBO J. 16 (19): 5966–76. doi:10.1093/emboj/16.19.5966. PMC 1170227
 . PMID 9312054.
. PMID 9312054. - ↑ Robles LD, Frost AR, Davila M, Hutson AD, Grizzle WE, Chakrabarti R (July 2002). "Down-regulation of Cdc6, a cell cycle regulatory gene, in prostate cancer". J. Biol. Chem. 277 (28): 25431–8. doi:10.1074/jbc.M201199200. PMID 12006585.
- ↑ Lau E, Tsuji T, Guo L, Lu SH, Jiang W (December 2007). "The role of pre-replicative complex (pre-RC) components in oncogenesis". FASEB J. 21 (14): 3786–94. doi:10.1096/fj.07-8900rev. PMID 17690155.