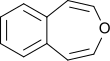
3-Benzoxepin
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3-benzoxepin | |||
| Other names
3-benzoxepine | |||
| Identifiers | |||
| 264-13-1 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 2892804 | ||
| PubChem | 3659427 | ||
| |||
| |||
| Properties | |||
| C10H8O | |||
| Molar mass | 144.17 g·mol−1 | ||
| Appearance | Yellow solid[1] | ||
| Melting point | 84 (83–84 °C;[2] 84 °C[1]) | ||
| soluble in apolar solvens, like Diethylether, Benzene, Tetrachloromethane,[3] alcohols, e.g. Methanol[2] | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
3-Benzoxepin is an annulated ring system with an aromatic benzene ring and a non-aromatic, unsaturated, oxygen-containing seven-membered heterocyclic oxepin. The first synthesis was described by Karl Dimroth and coworkers in 1961.[1] It is one of the three isomers of the benzoxepins.
Occurrence and synthesis
3-Benzoxepin itself is a non-natural compound, but the bicyclic ring system is part of the naturally occurring compounds perilloxin (I) from Perilla frutescens (variant acuta)[4] and tenual (II) and tenucarb (III) from Asphodeline tenuior.[5] Perilloxin inhibits the enzyme cyclooxygenase with an IC50 of 23.2 μM.[4] Non-steroidal anti-inflammatory drugs like aspirin and ibuprofen also work by inhibiting the cyclooxygenase enzyme family.[6]
Unsubstituted 3-benzoxepin can be synthesized through a double Wittig reaction from o-phthalaldehyde[7] with bis-(α,α′-triphenylphosphonium)-dimethylether-dibromide.[2] The latter compound can be synthesized from α,α′-dibromodimethyl ether (bis(bromomethyl)ether or BBME) which is accessible from hydrobromic acid, paraformaldehyde,[8] and triphenylphosphine. The reaction is performed in dry methanol with sodium methoxide, and the product is obtained in 55% yield.[1][3]
The compound can also be obtained through UV-irratiation of certain naphthalene derivatives such as 1,4-epoxy-1,4-dihydronaphthalene.[9]
It can also be obtained by photooxidation of 1,4-dihydronaphthalene, followed by pyrolysis of the formed hydroperoxides.[10]
The latter syntheses give 3-benzoxepins in low yields (4–6%).[9]
Properties
3-Benzoxepin is a bright yellow solid that crystallizes in platelets, with a smell similar to naphthalene. The material is soluble in apolar, organic solvents. Like naphthalene, it can be purified through sublimation. The solid is relatively acid-resistant, only under refluxing in concentrated, acidic alcohol solutions an unsaturated aldehyde is formed (likely an indene-3-aldehyde). Catalytic hydrogenation with a palladium catalyst results in 1,2,4,5-tetrahydro-3-benzoxepin.
References
- 1 2 3 4 Dimroth, K.; Pohl, G. (1961). "3-Benzoxepin". Angew. Chem. 73 (12): 436. doi:10.1002/ange.19610731215.
- 1 2 3 Dimroth, K.; Pohl, G.; Follmann, H. (1966). "Die Synthese von Derivaten des 3-Oxepins und des Furans durch eine zweifache Wittig-Reaktion". Chem. Ber. (in German). 99 (2): 634–641. doi:10.1002/cber.19660990238.
- 1 2 Rosowsky, A., ed. (1972). "II. Oxepin Ring Systems Containing Two Rings". Seven-Membered Heterocyclic Compounds Containing Oxygen and Sulfur. The Chemistry of Heterocyclic Compounds (in German). 26th. New York: Wiley-Interscience. p. 96. ISBN 0-471-38210-8.
- 1 2 Liu, J.-H.; Steigel, A.; Reininger, E.; Bauer, R. (2000). "Two new prenylated 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta". J. Nat. Prod. 63 (3): 403–405. doi:10.1021/np990362o.
- ↑ Ulubelen, A.; Tuzlaci, E.; Atilan, N. (1989). "Oxepine derivatives and anthraquinones from Asphodeline tenuior and A. taurica". Phytochem. 28 (2): 649–650. doi:10.1016/0031-9422(89)80076-7.
- ↑ Kester, M.; Karpa, K. D.; Vrana, K. E. (2011). "NSAIDs". Pharmacology. Elsevier's Integrated Review. Elsevier Health Sciences. pp. 165–166. ISBN 9780323074452.
- ↑ Bill, J. C.; Tarbell, D. S. (1954). "o-Phthalaldehyde". Org. Synth. 34: 82. doi:10.15227/orgsyn.034.0082.
- ↑ US patent 20040242799, Grabarnick, M. & Sasson, Y., "Process to bromomethylate aromatic compounds", published 2004-12-02, assigned to Grabarnick, M. and Sasson, Y.
- 1 2 Ziegler, G. R. (1969). "Mechanisms of photochemical reactions in solution. LVII. Photorearrangement of 1,4-epoxy-1,4-dihydronaphthalene to benz[f]oxepin". J. Am. Chem. Soc. 91 (2): 446–449. doi:10.1021/ja01030a040.
- ↑ Jeffrey, A. M.; Jerina, D. M. (1972). "Autoxidation of 1,4-dihydronaphthalene. Formation of 3-benzoxepin via pyrolysis of 2-hydroperoxy-1,2-dihydronaphthalene". J. Am. Chem. Soc. 94 (11): 4048–4049. doi:10.1021/ja00766a084.