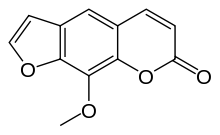
Methoxsalen
 | |
| Clinical data | |
|---|---|
| Trade names | Oxsoralen-Ultra, Uvadex, 8-mop, Oxsoralen |
| AHFS/Drugs.com | Consumer Drug Information |
| ATC code | D05AD02 (WHO) D05BA02 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | ~2 hours |
| Identifiers | |
| |
| CAS Number |
298-81-7 |
| PubChem (CID) | 4114 |
| DrugBank |
DB00553 |
| ChemSpider |
3971 |
| UNII |
U4VJ29L7BQ |
| KEGG |
D00139 |
| ChEBI |
CHEBI:18358 |
| ChEMBL |
CHEMBL416 |
| NIAID ChemDB | 001590 |
| ECHA InfoCard | 100.005.516 |
| Chemical and physical data | |
| Formula | C12H8O4 |
| Molar mass | 216.19 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Methoxsalen — also called xanthotoxin, marketed under the trade names Oxsoralen, Deltasoralen, Meladinine — is a drug used to treat psoriasis, eczema, vitiligo, and some cutaneous lymphomas in conjunction with exposing the skin to UVA light from lamps or sunlight. Methoxsalen modifies the way skin cells receive the UVA radiation, allegedly clearing up the disease. The dosage comes in 10 mg tablets, which are taken in the amount of 30 mg 75 minutes before a PUVA (psoralen + UVA) light treatment. Chemically, methoxsalen belongs to a class of organic natural molecules known as furanocoumarins. They consist of coumarin annulated with furan. It can also be injected and used topically.
Natural occurrences
Methoxsalen is extracted from Ammi majus, a plant of the family Apiaceae. The substance is also present in other Apiaceae as well as Rutaceae, for example bergamot oil which is used in many perfumes and aromatherapy oils. When these products are applied to skin and exposed to UVA radiation, the skin may turn brownish due to the phototoxic effects of methoxsalen present in the bergamot oil.[1] Most modern perfumes containing bergamot have the Methoxsalen removed.
Biosynthesis
The biosynthetic pathway is a combination of the shikimate pathway, which produces umbelliferone, and the mevalonate pathway.
Synthesis of umbelliferone
Umbelliferone is a phenylpropanoid and as such is synthesized from L-phenylalanine, which in turn is produced via the shikimate pathway. Phenylalanine is lysated into cinnamic acid, followed by hydroxylation by cinnamate 4-hydroxylase to yield 4-coumaric acid. The 4-coumaric acid is again hydroxylated by cinnamate/coumarate 2-hydroxylase to yield 2,4-dihydroxy-cinnamic acid (umbellic acid) followed by a bond rotation of the unsaturated bond adjacent to the carboxylic acid group. Finally an intramolecular attack from the hydroxyl group of C2' to the carboxylic acid group closes the ring and forms the lactone umbelliferone.
Synthesis of methoxsalen
The biosynthetic route then continues with the activation of dimethylallyl pyrophosphate (DMAPP), produced via the mevalonate pathway, to form a carbo-cation via the cleavage of the diphosphates. Once activated, the enzyme umbelliferone 6-prenyltransferase catalyzes a C-alkylation between DMAPP and umbelliferone at the activated position ortho to the phenol, yielding demethylsuberosin. This is then followed by a hydroxylation catalyzed by the enzyme marmesin synthase to yield marmesin. Another hydroxylation is catalyzed by psoralen synthase to yield psoralen. A third hydroxylation by the enzyme psoralen 8-monooxygenase yields xanthotoxol which is followed by a methylation via the enzyme xanthotoxol O-methyltransferase and S-adenosyl methionine to yield methoxsalen.[2]
Risks and side effects
Patients with high blood pressure or a history of liver problems are at risk for inflammation and irreparable damage to both liver and skin. The eyes must be protected from UVA radiation. Side effects include nausea, headaches, dizziness, and in rare cases insomnia. Methoxsalen has also been classified as an IARC Group 1 carcinogen (known to cause cancer) but is only cancerous when combined with UVA radiation.[3]
Cultural aspects
Author John Howard Griffin (1920–1980) used the chemical to darken his skin in order to investigate racial segregation in the American South. He wrote the book Black Like Me (1961) about his experiences.[4]
References
- ↑ "Bergamot". oilsandplants.com. Retrieved 2010-12-29.
- ↑ Dewick, P. M. (2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). John Wiley & Sons. pp. 161, 164–165.
- ↑ http://www.cancer.org/cancer/cancercauses/othercarcinogens/generalinformationaboutcarcinogens/known-and-probable-human-carcinogens
- ↑ Dead Like Me on snopes.com

