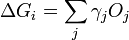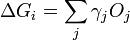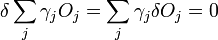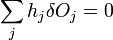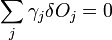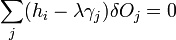Wulff construction
The Wulff construction is a method to determine the equilibrium shape of a droplet or crystal of fixed volume inside a separate phase (usually its saturated solution or vapor). Energy minimization arguments are used to show that certain crystal planes are preferred over others, giving the crystal its shape.
Theory
In 1878 Josiah Willard Gibbs proposed[1] that a droplet or crystal will arrange itself such that its surface Gibbs free energy is minimized by assuming a shape of low surface energy. He defined the quantity
Here 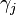 represents the surface (Gibbs free) energy per unit area of the
represents the surface (Gibbs free) energy per unit area of the 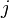 th crystal face and
th crystal face and 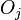 is the area of said face.
is the area of said face. 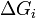 represents the difference in energy between a real crystal composed of
represents the difference in energy between a real crystal composed of  molecules with a surface and a similar configuration of
molecules with a surface and a similar configuration of  molecules located inside an infinitely large crystal. This quantity is therefore the energy associated with the surface. The equilibrium shape of the crystal will then be that which minimizes the value of
molecules located inside an infinitely large crystal. This quantity is therefore the energy associated with the surface. The equilibrium shape of the crystal will then be that which minimizes the value of 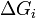 .
.
In 1901 Russian scientist George Wulff stated[2] (without proof) that the length of a vector drawn normal to a crystal face 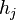 will be proportional to its surface energy
will be proportional to its surface energy 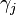 :
: 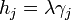 . The vector
. The vector 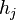 is the "height" of the
is the "height" of the 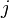 th face, drawn from the center of the crystal to the face; for a spherical crystal this is simply the radius. This is known as the Gibbs-Wulff theorem.
th face, drawn from the center of the crystal to the face; for a spherical crystal this is simply the radius. This is known as the Gibbs-Wulff theorem.
In 1953 Herring gave a proof of the theorem and a method for determining the equilibrium shape of a crystal, consisting of two main exercises. To begin, a polar plot of surface energy as a function of orientation is made. This is known as the gamma plot and is usually denoted as 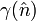 , where
, where 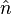 denotes the surface normal, e.g., a particular crystal face. The second part is the Wulff construction itself in which the gamma plot is used to determine graphically which crystal faces will be present. It can be determined graphically by drawing lines from the origin to every point on the gamma plot. A plane perpendicular to the normal
denotes the surface normal, e.g., a particular crystal face. The second part is the Wulff construction itself in which the gamma plot is used to determine graphically which crystal faces will be present. It can be determined graphically by drawing lines from the origin to every point on the gamma plot. A plane perpendicular to the normal 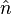 is drawn at each point where it intersects the gamma plot. The inner envelope of these planes forms the equilibrium shape of the crystal.
is drawn at each point where it intersects the gamma plot. The inner envelope of these planes forms the equilibrium shape of the crystal.
Proof
Various proofs of the theorem have been given by Hilton, Liebman, von Laue,[3] Herring,[4] and a rather extensive treatment by Cerf.[5] The following is after the method of R. F. Strickland-Constable.[6] We begin with the surface energy for a crystal
which is the product of the surface energy per unit area times the area of each face, summed over all faces. This is minimized for a given volume when
We then consider a small change in shape for a constant volume
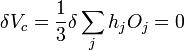 ,
,
which can be written as
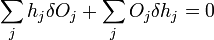 .
.
The second term must be zero, as it represents the change in volume [which does not make sense, as before the whole expression was supposed to be the change in volume] and we wish only to find the lowest surface energy at a constant volume (i.e., without adding or removing material). We are then given from above
and
which can be combined by a constant of proportionality as
The change in shape 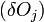 must be allowed to be arbitrary, which then requires that
must be allowed to be arbitrary, which then requires that 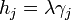 , which then proves Gibbs-Wulff Theorem.
, which then proves Gibbs-Wulff Theorem.
References
- ↑ Gibbs, Collected Works, 1928
- ↑ G Wulff, Zeitschrift fur Krystallographie und Mineralogie, 34, 5/6, pp 449-530, 1901.
- ↑ M von Laue Zeitschrift fur Kristallographie 105,2 pp:124-133, AUG 1943
- ↑ Herring Angewandte Chemie 63, 1 p: 34, 1953 http://onlinelibrary.wiley.com/doi/10.1002/ange.19530650106/pdf
- ↑ R Cerf: The Wulff Crystal in Ising and Percolation Models, Springer, 2006
- ↑ R. F. Strickland-Constable: Kinetics and Mechanism of Crystallization, page 77, Academic Press, 1968.