Wenxiang diagram
The Wenxiang diagram, also known as the Wenxiang graph, was proposed in 1997 for helping intuitively analyze the disposition of amphiphilic alpha helices in heteropolar environments[1] It is closely related to the earlier 2D diagram called a "helical wheel",[2] which is a slightly idealized projection of the Calphas down the helix axis, also with one-letter-code labels and color-coded symbols to show the sequence and its properties. This diagram is called “wenxiang” because of its shape that resembles the coil-like incense widely used in old China to repel mosquitoes, called 蚊香 and pronounced as "wenxiang".
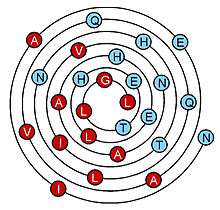
Because the wenxiang diagram is generated by a conical projection of a helix onto a plane, with the start (N-terminus) of the helix at the edge and the end toward the center, the location of each residue in a helix is not only defined by an angle around the diagram’s center, but is also defined by its radius from the center of the diagram in number of turns, which corresponds to its distance along the helix, in number of turns. Therefore, in principle, the wenxiang diagram can be used to represent an alpha-helix of any length. The example shown is quite long, with 7 turns and sequence NIAEAVQQLNHTIVNAAHELHETLGL; its hydrophobic side is in red and its hydrophilic side in blue.
Wenxiang diagrams have been used to study helix-helix interactions[3] and protein-protein interactions.[4][5]
References
- ↑ Chou KC, Zhang CT, Maggiora GM (May 1997). "Disposition of amphiphilic helices in heteropolar environments". Proteins. 28 (1): 99–108. doi:10.1002/(SICI)1097-0134(199705)28:1<99::AID-PROT10>3.0.CO;2-C. PMID 9144795.
- ↑ Schiffer M, Edmundson AB (March 1967). "Use of helical wheels to represent the structures of proteins and to identify segments with helical potential". Biophys. J. 7 (2): 121–35. Bibcode:1967BpJ.....7..121S. doi:10.1016/S0006-3495(67)86579-2. PMC 1368002
 . PMID 6048867.
. PMID 6048867. - ↑ Kurochkina N (May 2010). "Helix-helix interactions and their impact on protein motifs and assemblies". J. Theor. Biol. 264 (2): 585–92. doi:10.1016/j.jtbi.2010.02.026. PMID 20202472.
- ↑ Zhou GP (June 2011). "The disposition of the LZCC protein residues in wenxiang diagram provides new insights into the protein-protein interaction mechanism". J Theor Biol. 284 (1): 142–8. doi:10.1016/j.jtbi.2011.06.006. PMID 21718705.
- ↑ Zhou GP (2011). "The Structural Determinations of the Leucine Zipper Coiled-Coil Domains of the cGMP-Dependent Protein Kinase I alpha and its Interaction with the Myosin Binding Subunit of the Myosin Light Chains Phosphase". Protein & Peptide Letters. 18 (10): 966–978. doi:10.2174/0929866511107010966. PMID 21592084.