Water softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water is more compatible with soap and extends the lifetime of plumbing. Water softening is usually achieved using lime softening or ion-exchange resins.
Rationale
The presence of certain metal ions like calcium and magnesium principally as bicarbonates, chlorides, and sulfates in water causes a variety of problems.[1]
Hard water leads to the buildup of limescale, which can foul plumbing, and promote galvanic corrosion.[2] In industrial scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems.
The slippery feeling experienced when using soap with soft water occurs because soaps tend to bind to fats in the surface layers of skin, making soap molecules difficult to remove by simple dilution. In contrast, in hard-water areas the rinse water contains calcium or magnesium ions which form insoluble salts, effectively removing the residual soap from the skin but potentially leaving a coating of insoluble stearates on tub and shower surfaces, commonly called soap scum.[3]
Which of these effects is considered more or less desirable varies from person to person, and those who dislike the sliminess and difficulty of washing off soap caused by soft water may harden the water by adding chemicals such as baking soda, calcium chloride or magnesium sulphate.[4]

Methods
The most common means for removing water hardness rely on ion-exchange resin or reverse osmosis. Other approaches include precipitation methods and sequestration by the addition of chelating agents.
Ion-exchange resin devices
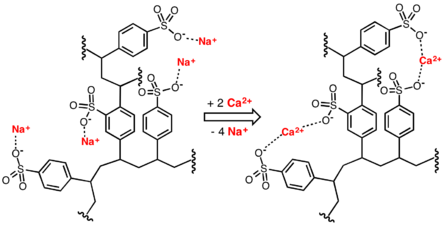
Conventional water-softening appliances intended for household use depend on an ion-exchange resin in which "hardness ions" - mainly Ca2+ and Mg2+ - are exchanged for sodium ions.[5] As described by NSF/ANSI Standard 44,[6] ion-exchange devices reduce the hardness by replacing magnesium and calcium (Mg2+ and Ca2+) with sodium or potassium ions (Na+ and K+)."

Ion exchange resins are organic polymers containing anionic functional groups to which the divalent cations (Ca++) bind more strongly than monovalent cations (Na+). Inorganic materials called zeolites also exhibit ion-exchange properties. These minerals are widely used in laundry detergents. Resins are also available to remove carbonate, bi-carbonate and sulphate ions which are absorbed and hydroxide ions released from the resin.
When all the available Na+ ions have been replaced with calcium or magnesium ions, the resin must be re-charged by eluting the Ca2+ and Mg2+ ions using a solution of sodium chloride or sodium hydroxide depending on the type of resin used.[7] For anionic resins, regeneration typically uses a solution of sodium hydroxide (lye) or potassium hydroxide. The waste waters eluted from the ion-exchange column containing the unwanted calcium and magnesium salts are typically discharged to the sewage system.
Lime softening
Lime softening is the process in which lime is added to hard water to make it softer.
Chelating agents
Chelators are used in chemical analysis, as water softeners, and are ingredients in many commercial products such as shampoos and food preservatives. Citric acid is used to soften water in soaps and laundry detergents. A commonly used synthetic chelator is ethylenediaminetetraacetic acid (EDTA).
Distillation and rain water
Since Ca2+ and Mg2+ exist as nonvolatile salts, they can be removed by distilling the water. Distillation is too expensive in most cases. Rainwater is soft because it is naturally distilled during the water cycle of evaporation, condensation and precipitation.[8]
Reverse osmosis
Reverse osmosis (RO) takes advantage of hydrostatic pressure gradients across a special membrane. The membrane has pores large enough to admit water molecules for passage; hardness ions such as Ca2+ and Mg2+ remain behind and are flushed away by excess water into a drain. The resulting soft water supply is free of hardness ions without any other ions being added. Membranes have a limited capacity, requiring regular replacement.
Non-chemical devices
Some manufacturers claim that their electronic devices affect the interaction of minerals with water so that the minerals do not bind to surfaces. Since these systems do not work by exchanging ions, like traditional water softeners do, one benefit claimed for the user is the elimination of the need to add salt to the system. While particle size reduction and plant growth promotion have been claimed, such systems do not remove minerals from the water itself. Rather, they can only alter the downstream effects that the mineral-bearing water would otherwise have. Examples are remediation of calcium scaling[9][10] and remediation of salt crusts in soil.[11] These systems do not fall within the term "water softening" but rather "water conditioning".
Similar claims for magnetic water treatment are not considered to be valid. For instance, no reduction of scale formation was found when such a magnet device was scientifically tested.[12]
Health effects
The CDC recommends limiting daily total sodium intake to 2,300 mg per day,[13] though the average American consumes 3,500 mg per day.[14] Because the amount of sodium present in drinking water—even after softening—does not represent a significant percentage of a person's daily sodium intake, the EPA considers sodium in drinking water to be unlikely to cause adverse health effects.[15]
For those who are on sodium-restricted diets, the use of a reverse osmosis system for drinking water and cooking water will remove sodium along with any other impurities which may be present. Potassium chloride can also be used as a regenerant instead of sodium chloride, although it is more costly. For people with impaired kidney function, however, elevated potassium levels, or hyperkalemia, can lead to complications such as cardiac arrhythmia.
Compared to reverse osmosis and distilled methods of producing soft water, hard water conveys some benefits to health by reducing the solubility of potentially toxic metal ions such as lead and copper, which are more soluble in soft water than in hard water.[16]
Environmental impact
Softened water (measured as residual sodium carbonate index) in which calcium and magnesium have been partly replaced by sodium is not suitable for irrigation use, as it tends to cause the development of alkali soils.[17] Non-chemical devices are often used in place of traditional water softening for this application.
See also
References
- ↑ The Editors of Encyclopædia Britannica. "Hard water". Encyclopædia Britannica. Retrieved 4 March 2015.
- ↑ Stephen Lower (July 2007). "Hard water and water softening". Retrieved 2007-10-08.
- ↑ "Archived copy". Archived from the original on 2011-08-17. Retrieved 2011-08-16.
- ↑ "Soft Water V. Hard Water In Plumbing, Pools And Hot Tubs Spas". Retrieved 2013-06-23.
- ↑ "Water Softeners". Canadian Mortgage and Housing Corporation. Retrieved 2010-01-29.
- ↑ Filtration Facts, September 2005, U.S. Environmental Protection Administration, pp. 6-7. Accessed 6 January 2013.
- ↑ "Ion Exchange Treatment of Drinking Water" (PDF). Des.nh.gov. 2009. Retrieved 2016-07-23.
- ↑ Bartram, edited by Jamie; Ballance, Richard (1996). Water quality monitoring : a practical guide to the design and implementation of freshwater quality studies and monitoring programmes (1st ed.). London: E & FN Spon. ISBN 0419223207.
- ↑ "Softening Alternatives". wcponline.com. Retrieved 2016-11-26.
- ↑ "Evaluation of Alternatives to Domestic Ion Exchange Water Softeners" (PDF). Arisawater.com. Retrieved 2016-07-23.
- ↑ "Testimonials". Hydrosmart.com.au. Retrieved 2016-07-23.
- ↑ Krauter, P. W.; Harrar, J. E.; Orloff, S. P.; Bahowick, S. M. (1 December 1996). "Test of a Magnetic Device for the Amelioration of Scale Formation at Treatment Facility D". Osti.gov.
- ↑ "Salt Home — DHDSP". Cdc.gov. Retrieved 2016-07-23.
- ↑ Layton, Lyndsey (20 April 2010). "FDA plans to limit amount of salt allowed in processed foods for health reasons". Washingtonpost.com.
- ↑ "Drinking Water Contaminant Candidate List (CCL) and Regulatory Determination | US EPA". Water.epa.gov. 2016-05-09. Retrieved 2016-07-23.
- ↑ "Common Water Quality Problems And Their Treatment" (PDF). Retrieved 2013-06-23.
- ↑ "Managing irrigation water quality" (PDF). Oregon State University. p. 12. Retrieved 2012-10-04.