Water cluster
In chemistry a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water.[1] These clusters have been found experimentally or predicted in silico in various forms of water; in ice, in crystal lattices and in bulk liquid water, the simplest one being the water dimer (H2O)2 . Shu et al. reported the images of water clusters of 100 micrometres.[2][3] Ongoing academic research is important because the realization that water manifests itself as clusters rather than an isotropic collection may help explain many anomalous water characteristics such as its highly unusual density temperature dependence. Water clusters are also implicated in the stabilization of certain supramolecular structures. So little is understood about water clusters in bulk water that it is considered one of the unsolved problems in chemistry.
Theoretical studies (in-silico structures)
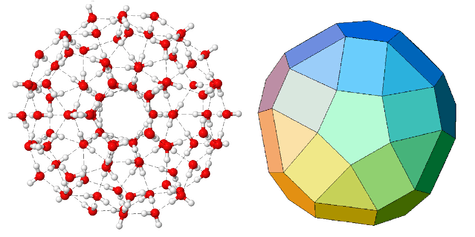
In-silico (see: water models), cyclic water clusters (H2O)n are found with n = 3 to 60.[4][5] Structures of water molecules with the highest resolution have been demonstrated in the studies of Richard Saykally of Berkeley College of Chemistry.[6] With increasing cluster size the oxygen to oxygen distance is found to decrease which is attributed to so-called cooperative many-body interactions: due to a change in charge distribution the H-acceptor molecule becomes a better H-donor molecule with each expansion of the water assembly. Many isomeric forms seem to exist for the hexamer: from ring, book, bag, cage, to prism shape with nearly identical energy. Two cage-like isomers exist for heptamers, and octamers are found either cyclic or in the shape of a cube. Even larger clusters are predicted: the fullerene-like cluster (H2O)28 is called the water buckyball and even for a 280 water molecule monster icosahedral network (with each water molecule coordinate to 4 others) there is found a local energy minimum. The 280 molecule icosahedral structure, which is 3 nm in diameter, consists of icosahedral shells with 280, 100 and 20 molecules (the 100 molecule structure is shown the figure above).[7][8] There is increased stability with the addition of each shell.[9] A look at the recent scientific literature may reveal good reviews on the studies of water clusters employing ab initio methods.[10][11] These clusters are also important for studying hydration phenomena at molecular level since they form the basic building blocks of the hydrated clusters.[12][13][14] There are theoretical models of water clusters of more than 700 water molecules by Martin Chaplin and Stanislav Zenin.[15][16] They have not been proven experimentally.
Experimental structures
Shu et al. observed water clusters under microscope. The experiments were conducted in two ways. One is making sodium chloride solutions and sampling water clusters from the solution and put the solution with water clusters on a glass slide under a microscope. The second method is to put a drop of Milli Q water on a glass slide under a microscope and put a grain of salt next to the water drop then push the salt grain inside the water drop. Under the microscope salt starts to dissolve and break into smaller salt particles. Some of the salt particles enter water clusters and reveals the appearance of water clusters.
The experimental observation[17][18] of water clusters requires sophisticated spectroscopic tools such as Far-infrared (FIR) vibration-rotation-tunneling (VRT) spectroscopy (an infrared spectroscopy technique). With water trapped in a liquid helium environment the hexamer is found to be a cyclic planar assembly but in the gas-phase the cage is found and in an organic host (water trapped in the crystal lattice of an organic compound) a conformation reminiscent of a cyclohexane chair conformation. Experiments combining IR spectroscopy with mass spectrometry reveal cubic configurations for clusters in the range W8-W10.
When the water is part of a crystal structure as in a hydrate, x-ray diffraction can be used. In a recent study the conformation of a water heptamer was determined (cyclic twisted nonplanar) using this method[19]
Experimental study of any supramolecular structures in bulk water is difficult because of their short lifetime: the hydrogen bonds are continually breaking and reforming at the timescales faster than 200 femtoseconds.[20]
Bulk water models
According to the so-called in silico method quantum cluster equilibrium (QCE) theory of liquids W8 clusters dominate the liquid water bulk phase followed by W5 and W6 clusters. In order to facilitate a water triple point the presence of a W24 cluster is invoked. In another model bulk water is built up from a mixture of hexamer and pentamer rings containing cavities capable of enclosing small solutes. In yet another model an equilibrium exists between a cubic water octamer and two cyclic tetramers. However, in spite of much model-making, no model yet has reproduced the experimentally-observed density maximum.[21][22]
See also
References
- ↑ Ralf Ludwig (2001). "Water: From Clusters to the Bulk". Angew. Chem. Int. Ed. 40 (10): 1808–1827. doi:10.1002/1521-3773(20010518)40:10<1808::AID-ANIE1808>3.0.CO;2-1. PMID 11385651.
- ↑ Shu, L., Obagbemi, I. J., Jegatheesan, V., Liyanaarachchi, S., Baskaran, K. (2015) Effect of multiple cations in the feed solution on the performance of forward osmosis, Desalination and Water Treatment, Vol. 54, pp845-852.
- ↑ Shu, L., Wu, S., Jegatheesan, V. (2013) Directly observe sodium chloride aggregates waltzing through dilute solutions, in ed., Shu, L., Jegatheesan, V., Pandey, A. Virkutyte, J., Djati Utomo, H. Solutions to Environmental Challenges through Innovation in Research, Asiatech, New Delhi. ISBN 81-87680-31-8.
- ↑ Fowler, P. W., Quinn, C. M., Redmond, D. B. (1991) Decorated fullerenes and model structures for water clusters, The Journal of Chemical Physics, Vol. 95, No 10, p. 7678.
- ↑ Ignatov, I., Mosin, O. V. (2013) Structural Mathematical Models Describing Water Clusters, Journal of Mathematical Theory and Modeling, Vol. 3, No 11, pp. 72-87.
- ↑ Keutsch, F. N. and Saykally, R. J. (2001) Water clusters: Untangling the mysteries of the liquid, one molecule at a time, PNAS, Vol. 98, № 19, pp. 10533–10540.
- ↑ Tokmachev, A.M., Tchougreeff, A.L., Dronskowski, R. (2010) Hydrogen-Bond Networks in Water Clusters: An Exhaustive Quantum-Chemical, European Journal of Chemical Physics And Physical Chemistry, Vol. 11, №2, pp. 384–388.
- ↑ Sykes, М. (2007) Simulations of RNA Base Pairs in a Nanodroplet Reveal Solvation-Dependent Stability, PNAS, Vol. 104, № 30, pp. 12336–12340.
- ↑ Loboda, Oleksandr; Goncharuk, Vladyslav (2010). "Theoretical study on icosahedral water clusters". Chemical Physics Letters. 484 (4–6): 144–147. Bibcode:2010CPL...484..144L. doi:10.1016/j.cplett.2009.11.025.
- ↑ S. Maheshwary; N. Patel; N Sathyamurthy; A. D. Kulkarni; S. R. Gadre (2001). "Structure and Stability of Water Clusters (H2O)n, n = 8-20: An Ab Initio Investigation". J. Phys. Chem. a. 105 (46): 10525. doi:10.1021/jp013141b.
- ↑ G. S. Fanourgakis; E. Aprà; W. A. de Jong; S. S. Xantheas (2005). "High-level ab initio calculations for the four low-lying families of minima of (H2O)20. II. Spectroscopic signatures of the dodecahedron, fused cubes, face-sharinbucky water g pentagonal prisms, and edge-sharing pentagonal prisms hydrogen bonding networks". J. Chem. Phys. 122 (13): 134304. Bibcode:2005JChPh.122m4304F. doi:10.1063/1.1864892. PMID 15847462.
- ↑ A. D. Kulkarni; S. R. Gadre; S. Nagase (2008). "Quantum chemical and electrostatic studies of anionic water clusters(H2O)n−". J. Mol. Str. Theochem. 851 (1-3): 213. doi:10.1016/j.theochem.2007.11.019.
- ↑ A. D. Kulkarni; K. Babu; L. J. Bartolotti; S. R. Gadre. (2004). "Exploring Hydration Patterns of Aldehydes and Amides: Ab Initio Investigations". J. Phys. Chem. A. 108 (13): 2492. doi:10.1021/jp0368886.
- ↑ A. D. Kulkarni; R. K. Pathak; L. J. Bartolotti. (2005). "Structures, Energetics, and Vibrational Spectra of H2O2···(H2O)n, n = 1−6 Clusters: Ab Initio Quantum Chemical Investigations". J. Phys. Chem. A. 109 (20): 4583. doi:10.1021/jp044545h.
- ↑ Chaplin, M. F. (2013) What is liquid water, Science in Society, Iss. 58, 41-45.
- ↑ Zenin, S. V.(2002)Water, Federal Center for Traditional Methods for Diagnostics and Treatment, Moscow
- ↑ C. J. Gruenloh; J. R. Carney; C. A. Arrington; T. S. Zwier; S. Y. Fredericks; K. D. Jordan (1997). "Infrared Spectrum of a Molecular Ice Cube: The S4 and D2d Water Octamers in Benzene-(Water)8". Science. 276 (5319): 1678. doi:10.1126/science.276.5319.1678.
- ↑ M. R. Viant; J. D. Cruzan; D. D. Lucas; M. G. Brown; K. Liu; R. J. Saykally (1997). "Pseudorotation in Water Trimer Isotopomers Using Terahertz Laser Spectroscopy". J. Phys. Chem. a. 101 (48): 9032. doi:10.1021/jp970783j.
- ↑ M. H. Mir; J. J. Vittal (2007). "Phase Transition Accompanied by Transformation of an Elusive Discrete Cyclic Water Heptamer to a Bicyclic (H2O)7 Cluster". Angew. Chem. Int. Ed. 46 (31): 5925–5928. doi:10.1002/anie.200701779. PMID 17577896.
- ↑ Smith, Jared D.; Christopher D. Cappa; Kevin R. Wilson; Ronald C. Cohen; Phillip L. Geissler; Richard J. Saykally (2005). "Unified description of temperature-dependent hydrogen-bond rearrangements in liquid water" (PDF). Proc. Natl. Acad. Sci. USA. 102 (40): 14171–14174. Bibcode:2005PNAS..10214171S. doi:10.1073/pnas.0506899102. PMC 1242322
 . PMID 16179387.
. PMID 16179387. - ↑ Borowski, Piotr; Jaroniec, Justyna; Janowski, Tomasz; Woliński, Krzysztof (2003). "Quantum cluster equilibrium theory treatment of hydrogen-bonded liquids: Water, methanol and ethanol". Molecular Physics. 101 (10): 1413. Bibcode:2003MolPh.101.1413B. doi:10.1080/0026897031000085083.
- ↑ Lehmann, S. B. C.; Spickermann, C.; Kirchner, B. (2009). "Quantum Cluster Equilibrium Theory Applied in Hydrogen Bond Number Studies of Water. 1. Assessment of the Quantum Cluster Equilibrium Model for Liquid Water". Journal of Chemical Theory and Computation. 5 (6): 1640. doi:10.1021/ct800310a.
- Water clusters at London South Bank University Link
- The Cambridge Cluster Database - Includes water clusters calculated with various water models and the water clusters explored with ab initio methods.