von Braun reaction
The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide.[1] An example is the reaction of dimethyl-α-naphthylamine:[2]
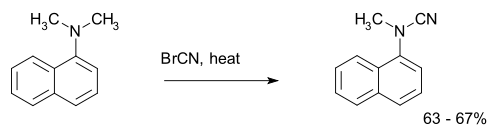
The reaction mechanism consists of two nucleophilic substitutions: the amine is the first nucleophile displacing the bromine atom which then acts as the second nucleophile.[3][4]
See also
References
- ↑ J. von Braun; K. Heider & E. Müller (1918). "Bromalkylierte aromatische Amine. II. Mitteilung". Chem. Ber. 51 (1): 273–282. doi:10.1002/cber.19180510132.
- ↑ Homer W. J. Cressman (1947). "N-Methyl-1-Naphthylcyanamide". Org. Synth. 27: 56. doi:10.15227/orgsyn.027.0056.
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ↑ Howard A. Hageman (1953). "The Von Braun Cyanogen Bromide Reaction". Organic Reactions. 7 (4): 198–262. doi:10.1002/0471264180.or007.04.
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.