Vitamin K2
Vitamin K2 or menaquinone (/ˌmɛnəˈkwɪnoʊn/) has nine related compounds, generally subdivided into the short-chain menaquinones (with MK-4 as the most important member) and the long-chain menaquinones, of which MK-7, MK-8 and MK-9 are nutritionally the most recognized.
Description
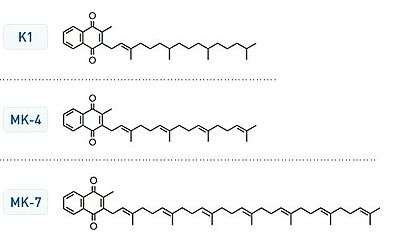
Vitamin K2, the main storage form in animals, has several subtypes, which differ in isoprenoid chain length. These vitamin K2 homologues are called menaquinones, and are characterized by the number of isoprenoid residues in their side chains. Menaquinones are abbreviated MK-n, where M stands for menaquinone, the K stands for vitamin K, and the n represents the number of isoprenoid side chain residues. For example, menaquinone-4 (abbreviated MK-4) has four isoprene residues in its side chain. Menaquinone-4 (also known as menatetrenone from its four isoprene residues) is the most common type of vitamin K2 in animal products since MK-4 is normally synthesized from vitamin K1 in certain animal tissues (arterial walls, pancreas, and testes) by replacement of the phytyl tail with an unsaturated geranylgeranyl tail containing four isoprene units, thus yielding menaquinone-4. This homolog of vitamin K2 may have enzyme functions distinct from those of vitamin K1.
Menaquinone-7 is different from MK-4 in that it is not produced by human tissue. MK-7 may be converted from phylloquinone (K1) in the colon by E. coli bacteria.[1] However, bacterially derived menaquinones (MK-7) appear to contribute minimally to overall vitamin K status.[2][3] MK-4 and MK-7 are both found in the United States in dietary supplements for bone health.
The U.S. Food and Drug Administration (FDA) has not approved any form of vitamin K for the prevention or treatment of osteoporosis; however, MK-4 has been shown to decrease the incidence of fractures up to 87%.[4] MK-4 (45 mg daily) has been approved by the Ministry of Health in Japan since 1995 for the prevention and treatment of osteoporosis.[5]
All K vitamins are similar in structure: they share a "quinone" ring, but differ in the length and degree of saturation of the carbon tail and the number of "side chains".[6] The number of side chains is indicated in the name of the particular menaquinone (e.g., MK-4 means that four molecular units - called isoprene units - are attached to the carbon tail) and this influences the transport to different target tissues.
Mechanism of action
The mechanism of action of vitamin K2 is similar to vitamin K1. Traditionally, K vitamins were recognized as the factor required for coagulation, but the functions performed by this vitamin group were revealed to be much more complex. K vitamins play an essential role as cofactor for the enzyme γ-glutamyl carboxylase, which is involved in vitamin K-dependent carboxylation of the gla domain in "Gla proteins" (i.e., in conversion of peptide-bound glutamic acid (Glu) to γ-carboxy glutamic acid (Gla) in these proteins).

Carboxylation of these vitamin K-dependent Gla-proteins, besides being essential for the function of the protein, is also an important vitamin recovery mechanism since it serves as a recycling pathway to recover vitamin K from its epoxide metabolite (KO) for reuse in carboxylation.
Several human Gla-containing proteins synthesized in several different types of tissues have been discovered:
- Coagulation factors (II, VII, IX, X), as well as anticoagulation proteins (C, S, Z). These Gla-proteins are synthesized in the liver and play an important role in blood homeo-stasis.
- Osteocalcin. This non-collagenous protein is secreted by osteoblasts and plays an essential role in the formation of mineral in bone.
- Matrix gla protein (MGP). This calcification inhibitory protein is found in numerous body tissues, but its role is most pronounced in cartilage and in arterial vessel walls.
- Growth arrest-specific protein 6 (GAS6). GAS6 is secreted by leucocytes and endothelial cells in response to injury and helps in cell survival, proliferation, migration, and adhesion.
- Proline-rich Gla-proteins (PRGP), transmembrane Gla-proteins (TMG), Gla-rich protein (GRP) and periostin; whose precise functions are still unexplored.
Absorption profile of different K vitamins
Vitamin K is absorbed along with dietary fat from the small intestine and transported by chylomicrons in the circulation. Most of vitamin K1 is carried by triacylglycerol-rich lipoproteins (TRL) and rapidly cleared by the liver; only a small amount is released into the circulation and carried by LDL and HDL. MK-4 is carried by the same lipoproteins (TRL, LDL, and HDL) and cleared fast as well. The long-chain menaquinones are absorbed in the same way as vitamin K1 and MK-4, but are efficiently redistributed by the liver in predominantly LDL (VLDL). Since LDL has a long half life in the circulation, these menaquinones can circulate for extended times resulting in higher bioavailability for extra-hepatic tissues as compared to vitamin K1 and MK-4. Accumulation of vitamin K in extra-hepatic tissues has direct relevance to vitamin K functions not related to hemostasis.[7]
Dietary sources and adequate intake
In 2012, Canadian health writer Kate Rhéaume-Bleue suggested the Recommended Daily Allowance (RDA) for K vitamins (range of 80-120 µg) might be too low.[8] Earlier suggestions in the scientific literature, which note that the RDA is based on hepatic (i.e. related to the liver) requirements only, date back as far as 1998.[9][10] This hypothesis is supported by the fact that the majority of the Western population exhibits a substantial fraction of undercarboxylated extra-hepatic proteins. Thus, complete activation of coagulation factors is satisfied, but there doesn’t seem to be enough vitamin K2 for the carboxylation of osteocalcin in bone and MGP in the vascular system.[11][12] Highest concentrations of vitamin K1 are found in green leafy vegetables, but significant concentrations are also present in non-leafy green vegetables, several vegetable oils, fruits, grains and dairy. In Europe and the USA 60%, or more, of total vitamin K1 intake is provided by vegetables, the majority by green leafy vegetables. National surveys reveal that K1 intakes vary widely. Intakes determined by weighed-dietary Intakes are similar in mainland Britain to the USA with average daily intakes of around 70–80 μg, which is less than the adequate intake for vitamin K. Apart from animal livers, the richest dietary source of long-chain menaquinones are fermented foods (from bacteria not moulds or yeasts) typically represented by cheeses (MK-8, MK-9) in Western diets and natto (MK-7) in Japan. Food frequency questionnaire-derived estimates of relative intakes in the Netherlands suggest that ~90% of total vitamin K intakes are provided by K1, ~7.5 % by MK-5 through to MK-9 and ~ 2.5% by MK-4. Most food assays measure only fully unsaturated menaquinones; accordingly cheeses have been found to contain MK-8 at 10–20 μg/100g and MK-9 at 35–55 μg/100 g.[13]
Dietary intake sources
Vitamin K2 is preferred by the extra-hepatic tissues (bone, cartilage, vasculature) and this may be produced as MK-4 by the animal from K1, or may be of bacterial origin (MK-7, MK-9, and other MK numbers). Discussion is ongoing as to what extent K2 produced by human intestinal bacteria contributes to daily vitamin K2 needs. If, however, intestinal bacterial supply was enough to supplement all tissues needing K2, we would not find high fractions of undercarboxylated Gla-proteins in human studies.
Menaquinone-4 is synthesized by animal tissues and is found in meat, eggs, and dairy products.[14] The MK-4 form of K2 is often found in relatively small quantities in meat and eggs. There are no substantial differences in MK-4 levels between wild game, free-range animals, and factory farm animals.[15]
Menaquinone-7 is synthesized by bacteria during fermentation and is found in fermented soybeans (natto), and in most fermented cheeses.[16] In natto, none of the vitamin K is from menaquinone-4, and in cheese only 2–7% is.[17] The richest source of natural K2 is the traditional Japanese dish natto[18] made of fermented soybeans and Bacillus subtilis, which provides an unusually rich source of K2 as long-chain MK-7: its consumption in Northern Japan has been linked to significant improvement in K vitamin's status and bone health in many studies. The intense smell and strong taste, however, make this soyfood a less attractive source of K2 for Westerners' tastes. Supplement food companies sell nattō extract, standardized for K2 content, in capsules. It is not known whether B. subtilis will produce K2 with other legumes (chickpeas, beans, lentils).
| Food 100 grams (3.5 oz) portion | Microgram (μg) | Proportion of vitamin K2 |
|---|---|---|
| Natto, cooked | 1,103.4[15] | (0% MK-4, 1% MK-5, 1% MK-6, 90% MK-7, 8% MK-8) |
| Goose liver pâté | 369.0[15] | (100% MK-4) |
| Australian emu oil | 360[19] | |
| Hard cheeses | 76.3[15] | (6% MK-4, 2% MK-5, 1% MK-6, 2% MK-7, 22% MK-8, 67% MK-9) |
| Soft cheeses | 56.5[15] | (6.5% MK-4, 0.5% MK-5, 1% MK-6, 2% MK-7, 20% MK-8, 70% MK-9) |
| Egg yolk, (Netherlands) | 32.1[15] | (98% MK-4, 2% MK-6) |
| Goose leg | 31.0[15] | (100% MK-4) |
| Grass-fed ghee and butter oil | 19.6-43.1, with the average 29.9[20] | |
| Curd cheeses | 24.8[15] | (2.6% MK-4, 0.4% MK-5, 1% MK-6, 1% MK-7, 20% MK-8, 75% MK-9) |
| Egg yolk (U.S.) | 15.5[21] | (100% MK-4) |
| Butter | 15.0[15] | (100% MK-4) |
| Chicken liver (raw) | 14.1[21] | (100% MK-4) |
| Chicken liver (pan-fried) | 12.6[21] | (100% MK-4) |
| Cheddar cheese (U.S.) | 10.2[21] | (6% MK-4, 94% other MK) |
| Meat franks | 9.8[21] | (100% MK-4) |
| Salami | 9.0[15] | (100% MK-4) |
| Chicken breast | 8.9[15] | (100% MK-4) |
| Chicken Leg | 8.5[15] | (100% MK-4) |
| Ground beef (medium fat) | 8.1[21] | (100% MK-4) |
| Luncheon meat | 7.7[15] | (100% MK-4) |
| Chicken liver (braised) | 6.7[21] | (100% MK-4) |
| Minced meat | 6.7[15] | (100% MK-4) |
| Calf's liver (pan-fried) | 6.0[21] | (100% MK-4) |
| Hot dog | 5.7[21] | (100% MK-4) |
| Bacon | 5.6[21] | (100% MK-4) |
| Whipping cream | 5.4[15] | (100% MK-4) |
| Sauerkraut | 4.8[15] | (8% MK-4, 17% MK-5, 31% MK-6, 4% MK-7, 17% MK-8, 23% MK-9) |
| Pork steak | 3.7[15] | (57% MK-4, 13% MK-7, 30% MK-8) |
| Duck breast | 3.6[15] | (100% MK-4) |
| Buttermilk | 2.5[15] | (8% MK-4, 4% MK-5, 4% MK-6, 4% MK-7, 24% MK-8, 56% MK-9) |
| Plaice | 2.2[15] | (9% MK-4, 14% MK-6, 4% MK-7, 73% MK-8) |
| Eel | 2.2[15] | (77% MK-4, 5% MK-6, 18% MK-7) |
| Fermented Cod Liver Oil | 1.8[20] | (69% MK-4, 18% MK-6, 6% MK-8, 7% MK-9) |
| Chocolate | 1.5[15] | (100% MK-4) |
| Beef | 1.1[15] | (100% MK-4) |
| Buckwheat bread | 1.1[15] | (100% MK-7) |
| Whole milk yogurt | 0.9[15] | (67% MK-4, 11% MK-5, 22% MK-8) |
| Whole milk | 0.9[15] | (89% MK-4, 11% MK-5) |
| Egg white | 0.9[15] | (100% MK-4) |
| Deer back | 0.7[15] | (100% MK-4) |
| Salmon | 0.5[15] | (100% MK-4) |
| Cow's liver (pan-fried) | 0.4[21] | (100% MK-4) |
| Mackerel | 0.4[15] | (100% MK-4) |
| Pork liver | 0.3[15] | (100% MK-4) |
| Rabbit leg | 0.1[15] | (100% MK-4) |
| Skim milk yogurt | 0.1[15] | (100% MK-8) |
Prawns, herring, kale, spinach, broccoli, green peas, banana, apple, orange, margarine, corn oil, sunflower oil, olive oil, rye bread, wheat bread, sourdough bread, and tea contain vitamin K1 but not vitamin K2. Skim milk and coffee do not contain any vitamin K.[15]
Anticoagulants and K2 supplementation
Recent studies found a clear association between long-term anticoagulant treatment (OAC) and reduced bone quality due to reduction of active osteocalcin. OAC might lead to an increased incidence of fractures, reduced bone mineral density/bone mineral content, osteopenia, and increased serum levels of undercarboxylated osteocalcin.[22] Bone mineral density was significantly lower in stroke patients with long-term warfarin treatment compared to untreated patients and osteopenia was probably an effect of warfarin-interference with vitamin K recycling.[23] Furthermore, OAC is often linked to an undesired soft-tissue calcification in both children and adults.[24][25] This process has been shown to be dependent upon the action of K vitamins. Vitamin K deficiency results in undercarboxylation of MGP. Vascular calcification was shown to appear in warfarin-treated experimental animals within two weeks.[26] Also in humans on OAC treatment, two-fold more arterial calcification was found as compared to patients not receiving vitamin K antagonists.[27][28] Among consequences of anticoagulant treatment: increased aortic wall stiffness, coronary insufficiency, ischemia, and even heart failure. Arterial calcification might also contribute to systolic hypertension and ventricular hypertrophy.[29][30] Coumarins, by interfering with vitamin K metabolism, might also lead to an excessive calcification of cartilage and tracheobronchial arteries.
Anticoagulant therapy is usually instituted to avoid life-threatening diseases and a high vitamin K intake interferes with the anticoagulant effect. Patients on warfarin (Coumadin) treatment, or treatment with other vitamin K antagonist drugs, are therefore advised not to consume diets rich in K vitamins. However, the latest research proposed to combine vitamins K with OAC to stabilize the INR (International normalized ratio, a laboratory test measure of blood coagulation).
Toxicity
There is no known toxicity associated with high doses of menaquinones (vitamin K2). A point of concern is however the potential interference of K vitamins with OAC treatment: individuals taking anticoagulant medications, such as warfarin (coumarins), should consult their doctor before taking Vitamin K2. Unlike the other fat-soluble vitamins, vitamin K is not stored in any significant quantity in the liver; therefore toxic level is not a described problem. All data available at this time demonstrate that vitamin K has no adverse effects in healthy subjects. The recommendations for the daily intake of vitamin K, as issued recently by the Institute of Medicine, also acknowledge the wide safety margin of vitamin K: "A search of the literature revealed no evidence of toxicity associated with the intake of either K1 or K2". Animal models involving rats, if generalizable to humans, show that MK-7 is well-tolerated.[31]
References
- ↑ Vermeer, C; Braam L (2001). "Role of K vitamins in the regulation of tissue calcification". Journal of bone and mineral metabolism. 19 (4): 201–206. doi:10.1007/s007740170021. PMID 11448011.
- ↑ Suttie, JW (1995). "The importance of menaquinones in human nutrition". Annual Review of Nutrition. 15: 399–417. doi:10.1146/annurev.nu.15.070195.002151. PMID 8527227.
- ↑ Weber, P (2001). "Vitamin K and bone health". Nutrition. 17 (10): 880–887. doi:10.1016/S0899-9007(01)00709-2. PMID 11684396.
- ↑ Sato, Y; Kanoko T; Satoh K; Iwamoto J (2005). "Menatetrenone and vitamin D2 with calcium supplements prevent nonvertebral fracture in elderly women with Alzheimer's disease". Bone. 36 (1): 61–8. doi:10.1016/j.bone.2004.09.018. PMID 15664003.
- ↑ Iwamoto, I; Kosha S; Noguchi S-i (1999). "A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy". Maturitas. 31 (2): 161–164. doi:10.1016/S0378-5122(98)00114-5. PMID 10227010.
- ↑ Shearer MJ.2003 in Physiology. Elsevier Sciences LTD. 6039-45.
- ↑ Martin J. Shearer, Paul Newman. Metabolism and cell biology of vitamin K. Thromb Haemost 2008
- ↑ Kate Rhéaume-Bleue, Vitamin K2 and the Calcium Paradox. Mississaugua: Wiley, 2012, p. 74.
- ↑ Booth SL, Suttie JW. Dietary intake and adequacy of K vitamins. J Nutr. 1998;128(5):785-8.
- ↑ Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570(1):27-32.
- ↑ Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18(3):251-9.
- ↑ Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD. Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J Bone Miner Res. 1991;6(11):1211-6.
- ↑ Shearer N, Metabolism and cell biology of vitamin K. Thromb Haemost. 2008
- ↑ Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL (2006). "Vitamin k contents of meat, dairy, and fast food in the U.S. Diet". J. Agric. Food Chem. 54 (2): 463–7. doi:10.1021/jf052400h. PMID 16417305.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Schurgers, Leon J.; Vermeer, Cees (November 2000). "Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations". Haemostasis. 30 (6): 298–307. Retrieved 12 October 2016.
- ↑ Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M (2000). "Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and gamma-carboxylated osteocalcin concentration in normal individuals". J. Bone Miner. Metab. 18 (4): 216–22. doi:10.1007/s007740070023. PMID 10874601.
- ↑ "On the Trail of the Elusive X-Factor: Vitamin K2 Revealed".
- ↑ Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H; Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of K vitamins2: possible implications for hip-fracture risk; Nutrition; 2001; 17(4): 315-321.
- ↑ "Factsheet - Vitamin K2" (PDF). emutracks.com.au.
- 1 2 "Hook Line and Stinker" (PDF).
- 1 2 3 4 5 6 7 8 9 10 11 Rhéaume-Bleue, Kate (August 27, 2013). Vitamin K2 and the Calcium Paradox: How a Little-Known Vitamin Could Save Your Life. Harper. pp. 66–67. ISBN 0062320041.
- ↑ Caraballo PJ, Gabriel SE, Castro MR, Atkinson EJ, Melton LJ 3rd. Changes in bone density after exposure to oral anticoagulants: a meta-analysis.Osteoporos Int. 1999;9(5):441-8.
- ↑ Sato Y, Honda Y, Kunoh H, Oizumi K. Long-term oral anticoagulation reduces bone mass in patients with previous hemispheric infarction and nonrheumatic atrial fibrillation. Stroke. 1997;28(12):2390-4.
- ↑ Barnes C, Newall F, Ignjatovic V, Wong P, Cameron F, Jones G, Monagle P. Reduced bone density in children on long-term warfarin. Pediatr Res. 2005;57(4):578-81.
- ↑ Hawkins D, Evans J. Minimising the risk of heparin-induced osteoporosis during pregnancy. Expert Opin Drug Saf. 2005;4(3):583-90
- ↑ Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18(9):1400-7.
- ↑ Schurgers LJ, Aebert H, Vermeer C, Bültmann B, Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004 15;104(10):3231-2.
- ↑ Koos R, Mahnken AH, Mühlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kühl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96(6):747-9.
- ↑ Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-43.
- ↑ Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663-9.
- ↑ Pucaj et al. (Sep 2011) "Safety and toxicological evaluation of a synthetic vitamin K2, menaquinone-7", Toxicology Mechanisms and Methods Vol.21 No.7 pp.520–532