Viscose
Viscose is both a semi-synthetic fiber, formerly called viscose rayon, or rayon and a solution of cellulose xanthate. The latter is produced by treating dissolving pulp with aqueous sodium hydroxide and carbon disulfide which is used to spin the viscose rayon fiber. Byproducts of the production process include sodium thiocarbonate, sodium carbonate, and sodium sulfide.[1] Viscose rayon fiber is a soft fiber commonly used in dresses, linings, shirts, shorts, coats, jackets, and other outerwear. It is also used in industrial yarns (tyre cord), upholstery and carpets, and in the casting of Cellophane.
Manufacture
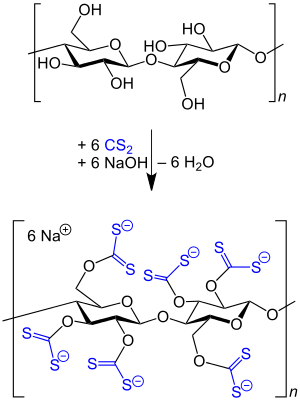

Viscose rayon is a fiber of regenerated cellulose; it is structurally similar to cotton but may be produced from a variety of plants such as soy, bamboo, and sugar cane. Cellulose is a linear polymer of β-D-glucose units with the empirical formula (C6H10O5)n.[3] To prepare viscose, dissolving pulp is treated with aqueous sodium hydroxide (typically 16-19% w/w) to form "alkali cellulose," which has the approximate formula [C6H9O4-ONa]n. The alkali cellulose is then treated with carbon disulfide to form sodium cellulose xanthate.[4]
- [C6H9O4-ONa]n + nCS2 → [C6H9O4-OCS2Na]n
The higher the ratio of cellulose to combined sulfur, the lower the solubility of the cellulose xanthate. The xanthate is dissolved in aqueous sodium hydroxide (typically 2-5% w/w) and allowed to depolymerize to a desired extent, indicated by the solution's viscosity. The rate of depolymerization (ripening or maturing) depends on temperature and is affected by the presence of various inorganic and organic additives, such as metal oxides and hydroxides.[4] Air also affects the ripening process since oxygen causes depolymerization.[5]
Rayon fiber is produced from the ripened solutions by treatment with a mineral acid, such as sulfuric acid. In this step, the xanthate groups are hydrolyzed to regenerate cellulose and release dithiocarbonic acid that later decomposes to carbon disulfide and water:[1]
- [C6H9O4-OCS2Na]2n + nH2SO4 → [C6H9O4-OH]2n +2nCS2 + nNa2SO4
- H2COS2 → H2O + CS2
Aside from regenerated cellulose, acidification gives hydrogen sulfide, sulfur, and carbon disulfide. The thread made from the regenerated cellulose is washed to remove residual acid. The sulfur is then removed by the addition of sodium sulfide solution and impurities are oxidized by bleaching with sodium hypochlorite solution.[4]
Pollution
The use of viscose is declining, in part because of the environmental costs of its production. Instead, rayon may be manufactured using the Lyocell process, which uses N-Methylmorpholine N-oxide as the solvent and produces little waste product, making it relatively eco-friendly.
History
French scientist and industrialist Hilaire de Chardonnet (1838–1924)— who invented the first artificial textile fiber, artificial silk—created viscose. British scientists Charles Frederick Cross and Edward John Bevan took out British patent no. 8,700, "Improvements in Dissolving Cellulose and Allied Compounds" in May, 1892.[6] In 1893 they formed the Viscose Syndicate to grant licences, and in 1896 formed the British Viscoid Co. Ltd. to exploit the process.[7]
Products made from viscose
- Art silk
- Cellophane
- Rayon
- Sausage casings
- Synthetic velvet
References
- 1 2 Wyss, George de (1 October 1925). "The Ripening of Viscose.". Industrial & Engineering Chemistry. 17 (10): 1043–1045. doi:10.1021/ie50190a018.
- ↑ Siegfried Hauptmann: Organische Chemie, 2. durchgesehene Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 652, ISBN 3-342-00280-8.
- ↑ Booth, Gerald (2000). Dyes, General Survey. Wiley-VCH. doi:10.1002/14356007.a09_073.
- 1 2 3 Wheeler, Edward (1928). The Manufacture of Artificial Silk With Special Reference to the Viscose Process. New York: D. Van Nostrand company.
- ↑ Bartell, F. E.; Cowling, Hale (1 May 1942). "Depolymermiation of Cellulose in Viscose Production". Industrial & Engineering Chemistry. 34 (5): 607–612. doi:10.1021/ie50389a017.
- ↑ Day, Lance; Ian McNeil (1998). Biographical Dictionary of the History of Technology. Taylor & Francis. p. 113. ISBN 0415193990.
- ↑ Woodings, Calvin R. "A Brief History of Regenerated Cellulosic Fibres". WOODINGS CONSULTING LTD. Retrieved 26 May 2012.
External links
- "Viscose". Plastiquarian.com. Archived from the original on 2008-06-25.