Viral entry
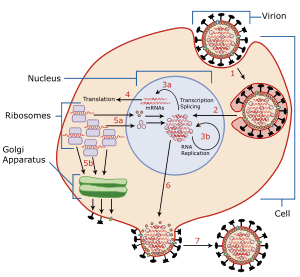 |
Viral entry is the earliest stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral material into the cell. The major steps involved in viral entry are shown below.[1] Despite the variation among viruses, there are several shared generalities concerning viral entry.
Reducing cellular proximity
A virus floating around an enclosed space with possible host cells faces a large hurdle, the thermodynamics of diffusion. Because neutrally charged objects do not naturally clump around each other, the virus must find a way to move even near a host cell. It does this by attachment -- or adsorption --- onto a susceptible cell; a cell which holds a receptor that the virus can bind to. The receptors on the viral envelope effectively become connected to complementary receptors on the cell membrane. This attachment causes the two membranes to remain in mutual proximity, favoring further interactions between surface proteins. This is also the first requisite that must be satisfied before a cell can become infected. Satisfaction of this requisite makes the cell susceptible. Viruses that exhibit this behavior include many enveloped viruses such as HIV and Herpes simplex virus
This basic idea extends to viruses that do not contain an envelope. Well studied examples are the viruses that infect bacteria, known as bacteriophages or simply phages. Typical phages have long tails used to attach to receptors on the bacterial surface.
Overview
Prior to entry, a virus must attach to a host cell. Attachment is achieved when receptors on the capsid or viral envelope become connected to complementary receptor proteins on the cell membrane of the target cell. A virus must now enter the cell, which is covered by a phospholipid bilayer, a cell's natural barrier to the outside world. The process by which this barrier is breached depends upon the virus. Types of entry are:
- Membrane Fusion or Hemifusion State: The cell membrane is punctured and made to further connect with the unfolding viral envelope.
- Endocytosis: The host cell takes in the viral particle through the process of endocytosis, essentially engulfing the virus like it would a food particle.
- Viral Penetration: The viral capsid or genome is injected into the host cell's cytoplasm.
Through the use of green fluorescent protein (GFP), virus entry and infection can be visualized in real-time. Once a virus enters a cell, replication is not immediate and indeed takes some time (seconds to hours).[2][3]
Entry via Membrane Fusion
.svg.png)
The most well-known example is through membrane fusion. In viruses with a viral envelope, viral receptors attach to the receptors on the surface of the cell and secondary receptors may be present to initiate the puncture of the membrane or fusion with the host cell. Following attachment, the viral envelope fuses with the host cell membrane, emptying the now-bare virus into the cell. In essence, the virus's envelope "blends" with the host cell membrane, releasing its contents into the cell. Obviously, this can only be done with viruses that have an envelope (examples of such enveloped viruses include HIV and herpes simplex virus.[4])
Entry via Endocytosis
.svg.png)
Viruses with no viral envelope enter the cell through endocytosis; they are ingested by the host cell through the cell membrane. In essence, the virus tricks the cell into thinking that the virus knocking at the door is nothing more than nutrition or harmless goods. A cell, which naturally takes in resources from the environment by attaching goods onto surface receptors and bringing them into the cell, will engulf the virus. Once inside the cell, the virus must now break out of the vesicle by which it was taken up in order to gain access to the cytoplasm. Examples include the poliovirus, Hepatitis C virus[5] and Foot-and-mouth disease virus.[6]
Many enveloped viruses also enter the cell through endocytosis. Entry via the endosome guarantees low pH and exposure to proteases which are needed to open the viral capsid and release the genetic material inside. Further, endosomes transport the virus through the cell and ensure that no trace of the virus is left on the surface, which could be a substrate for immune recognition. [7]
Entry via Genetic Injection
A third and more specific example, is by simply attaching to the surface of the cell via receptors on the cell, and injecting only its genome into the cell, leaving the rest of the virus on the surface. This is restricted to viruses in which only the gene is required for infection of a cell (most positive-sense, single-stranded RNA viruses because they can be immediately translated) and further restricted to viruses that actually exhibit this behavior. The best studied example includes the bacteriophages; for example, when the tail fibers of the T2 phage land on a cell, its central sheath pierces the cell membrane and the phage injects DNA from the head capsid directly into the cell.[8]
Aftermath
Once a virus is in a cell, it will activate formation of proteins (either by itself or using the host) to gain full control of the host cell, if it is able to. Control mechanisms include the suppression of intrinsic cell defenses, suppression of cell signaling and suppression of host cellular transcription and translation. Often, it is these cytotoxic effects that lead to the death and decline of a cell infected by a virus.
A cell is classified as susceptible to a virus if the virus is able to enter the cell. After the introduction of the viral particle, unpacking of the contents (viral proteins in the tegument and the viral genome via some form of nucleic acid) occurs as preparation of the next stage of viral infection: viral replication.
References
- ↑ Subramanian RP, Geraghty RJ (20 Feb 2007). "Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B". Proceedings of the National Academy of Sciences, USA. 104 (8): 2903–8. doi:10.1073/pnas.0608374104. PMC 1815279
 . PMID 17299053.
. PMID 17299053. - ↑ Lakadamyali, Melike; Michael J. Rust; Hazen P. Babcock; Xiaowei Zhuang (2003). "Visualizing infection of individual influenza viruses". Proceedings of the National Academy of Sciences of the United States of America. 100 (16): 9280–9285. doi:10.1073/pnas.0832269100. PMC 170909
 . PMID 12883000. Retrieved 2009-05-21.
. PMID 12883000. Retrieved 2009-05-21. - ↑ Joo, K-I; P Wang (2008-05-15). "Visualization of Targeted Transduction by Engineered Lentiviral Vectors". Gene Ther. 15 (20): 1384–1396. doi:10.1038/gt.2008.87. ISSN 0969-7128. PMC 2575058
 . PMID 18480844.
. PMID 18480844. - ↑ Campadelli-Fiume G, Amasio M, Avitabile E, Cerretani A, Forghieri C, Gianni T, Menotti L. "The multipartite system that mediates entry of herpes simplex virus into the cell." Rev Med Virol. 2007 Sep-Oct;17(5):313-26. Review.
- ↑ Helle F, Dubuisson J. "Hepatitis C virus entry into host cells." Cell Mol Life Sci. 2007 Oct 4
- ↑ N.J. Dimmock et al. "Introduction to Modern Virology, 6th edition." Blackwell Publishing, 2007.
- ↑ Howley, Peter M; Knipe, David M "Fields Virology" Lippincott Williams & Williams 2013
- ↑ Sebestyén MG, Budker VG, Budker T, Subbotin VM, Zhang G, Monahan SD, Lewis DL, Wong SC, Hagstrom JE, Wolff JA. "Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules." J Gene Med. 2006 Jul;8(7):852-73.