Vinyltestosterone
 | |
| Identifiers | |
|---|---|
| |
| Synonyms |
Ethenyltestosterone; 17α-Ethenyltestosterone |
| CAS Number | 1235-98-9 |
| PubChem (CID) | 222286 |
| ChemSpider | 192964 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.4617 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
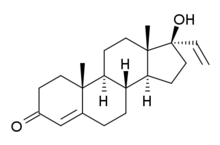
Vinyltestosterone, or 17α-vinyltestosterone, also known as 17α-vinylandrost-4-en-17β-ol-3-one, as well as 17α-hydroxypregna-4,20-dien-3-one, is a synthetic, steroidal androgenic-anabolic steroid (AAS) that was never marketed.[1][2] It is a relatively weak AAS.[2][3][4] In one study, vinyltestosterone showed approximately one-third and one-fifth of the respective androgenic and anabolic activity of other AAS such as nandrolone, methyltestosterone, and ethyltestosterone in castrated male rats, whereas ethisterone showed almost no androgenic and anabolic activity (only 1/20th the anabolic potency of vinyltestosterone).[3] Additionally, in women with metastatic breast cancer, vinyltestosterone was found to be ineffective in treating the disease (unlike other AAS such as testosterone propionate or fluoxymesterone)[5] and produced little or no virilization in the women at a dosage of 100 mg intramuscularly three times per week.[4][6] Aside from vinyltestosterone itself, two 19-nortestosterone derivatives of vinyltestosterone, norvinisterone (17α-vinyl-19-nortestosterone) and norgesterone (17α-vinyl-δ5(10)-19-nortestosterone), have, in contrast, been marketed.[7] They are used as progestins for female hormonal contraception, rather than as AAS.[7]
See also
References
- ↑ R.A. Hill; H.L.J. Makin; D.N. Kirk; G.M. Murphy (23 May 1991). Dictionary of Steroids. CRC Press. pp. 226–. ISBN 978-0-412-27060-4. Cite uses deprecated parameter
|coauthors=(help) - 1 2 LEWIS RA, DeMAJO S, ROSEMBERG E (1949). "The effects of 17-vinyl testosterone upon the rat adrenal". Endocrinology. 45 (6): 564–70. doi:10.1210/endo-45-6-564. PMID 15402199.
- 1 2 SAUNDERS FJ, DRILL VA (1956). "The myotrophic and androgenic effects of 17-ethyl-19-nortestosterone and related compounds". Endocrinology. 58 (5): 567–72. doi:10.1210/endo-58-5-567. PMID 13317831.
- 1 2 SCHEDL HP, DELEA C, BARTTER FC (1959). "Structure-activity relationships of anabolic steroids: role of the 19-methyl group". J. Clin. Endocrinol. Metab. 19: 921–35. doi:10.1210/jcem-19-8-921. PMID 14442516.
17α-Vinyltestosterone in large doses manifested weak myotropic and androgenic activities in castrated male rats (1) and proved to be ineffective in the therapy of patients with metastatic breast cancer, in whom it had little or no virilizing effect at a dosage level of 100 mg. intramuscularly three times a week (18).
- ↑ Gregory Pincus; Erwin P. Vollmer (17 September 2013). Biological Activities of Steroids in Relation to Cancer: Proceedings of a Conference Sponsored by the Cancer Chemotherapy National Service Center, National Cancer Institute, National Institutes of Health, U. S. Department of Health, Education and Welfare. Elsevier Science. pp. 26–. ISBN 978-1-4832-7057-9.
- ↑ SEGALOFF A, GORDON DL, HORWITT BN, MURISON PJ, SCHLOSSER JV (1955). "Hormonal therapy in cancer of the breast. X. The effect of vinyltestosterone therapy on clinical course and hormonal excretion". Cancer. 8 (5): 903–5. PMID 13261042.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887, 889. ISBN 978-1-4757-2085-3.