Vapour pressure of water
The vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. At higher pressures water would condense. The water vapor pressure is the partial pressure of water vapor in any gas mixture in equilibrium with solid or liquid water. As for other substances, water vapor pressure is a function of temperature and can be determined with Clausius–Clapeyron relation.
| T, °C | T, °F | P, kPa | P, torr | P, atm |
|---|---|---|---|---|
| 0 | 32 | 0.6113 | 4.5851 | 0.0060 |
| 5 | 41 | 0.8726 | 6.5450 | 0.0086 |
| 10 | 50 | 1.2281 | 9.2115 | 0.0121 |
| 15 | 59 | 1.7056 | 12.7931 | 0.0168 |
| 20 | 68 | 2.3388 | 17.5424 | 0.0231 |
| 25 | 77 | 3.1690 | 23.7695 | 0.0313 |
| 30 | 86 | 4.2455 | 31.8439 | 0.0419 |
| 35 | 95 | 5.6267 | 42.2037 | 0.0555 |
| 40 | 104 | 7.3814 | 55.3651 | 0.0728 |
| 45 | 113 | 9.5898 | 71.9294 | 0.0946 |
| 50 | 122 | 12.3440 | 92.5876 | 0.1218 |
| 55 | 131 | 15.7520 | 118.1497 | 0.1555 |
| 60 | 140 | 19.9320 | 149.5023 | 0.1967 |
| 65 | 149 | 25.0220 | 187.6804 | 0.2469 |
| 70 | 158 | 31.1760 | 233.8392 | 0.3077 |
| 75 | 167 | 38.5630 | 289.2463 | 0.3806 |
| 80 | 176 | 47.3730 | 355.3267 | 0.4675 |
| 85 | 185 | 57.8150 | 433.6482 | 0.5706 |
| 90 | 194 | 70.1170 | 525.9208 | 0.6920 |
| 95 | 203 | 84.5290 | 634.0196 | 0.8342 |
| 100 | 212 | 101.3200 | 759.9625 | 1.0000 |
Approximation formula
The saturated vapor pressure of water may be approximated by the following relations (in order of increasing accuracy):
- Using the Antoine equation
- where the temperature T is in degrees Celsius and the vapor pressure P is in torr. The constants are given as
A B C Tmin, °C Tmax, °C 8.07131 1730.63 233.426 1 99 8.14019 1810.94 244.485 100 374
Graphical pressure dependency on temperature
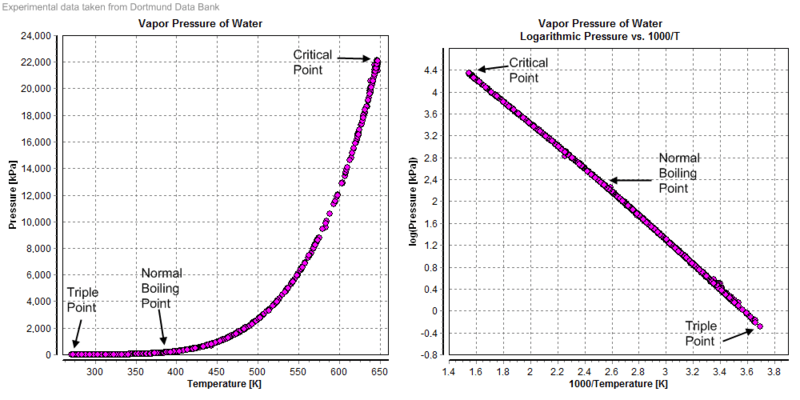
Vapour pressure diagrams of water; data taken from Dortmund Data Bank. Graphics shows triple point, critical point and boiling point of water.
See also
- Dew point
- Gas laws
- Molar mass
- Goff-Gratch equation
- Antoine equation
- Arden Buck equation
- Lee–Kesler method
References
- ↑ David R. Lide, ed. (2005). CRC Handbook of Chemistry and Physics. Boca Raton, Florida: CRC Press. p. 6-8.
Further reading
- http://web.mit.edu/seawater/ MIT page of seawater properties, with Matlab, EES and Excel VBA library routines
- Garnett, Pat; Anderton, John D; Garnett, Pamela J (1997). Chemistry Laboratory Manual For Senior Secondary School. Longman. ISBN 0-582-86764-9.
- Murphy, D. M. and Koop, T. (2005): Review of the vapour pressures of ice and supercooled water for atmospheric applications, Quarterly Journal of the Royal Meteorological Society 131(608): 1539–1565. doi:10.1256/qj.04.94
- James G. Speight : Lange's Handbook of Chemistry ISBN 0071432205
External links
This article is issued from Wikipedia - version of the 9/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.