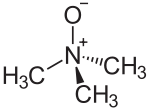
Trimethylamine N-oxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
trimethylamine oxide | |
| Preferred IUPAC name
N,N-dimethylmethanamine oxide | |
| Other names
trimethylamine oxide, TMAO, TMANO | |
| Identifiers | |
| 1184-78-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15724 |
| ChemSpider | 1113 |
| ECHA InfoCard | 100.013.341 |
| KEGG | C01104 |
| PubChem | 1145 |
| UNII | FLD0K1SJ1A |
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colorless solid |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) (hydrate: 96 °C) |
| good | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Trimethylamine N-oxide (TMAO) is the organic compound in the class of amine oxides with the formula (CH3)3NO. This colorless solid is usually encountered as the dihydrate. It is a product of the oxidation of trimethylamine, a common metabolite in animals. It is an osmolyte found in saltwater fish, sharks, rays, molluscs, and crustaceans. It is a protein stabilizer that may serve to counteract urea, the major osmolyte of sharks, skates and rays. It is also higher in deep-sea fishes and crustaceans, where it may counteract the protein-destabilizing effects of pressure.[1] TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.
Synthesis
TMAO can be synthesized from trimethylamine by treatment with hydrogen peroxide:[2]
- H2O2 + (CH3)3N → H2O + (CH3)3NO
TMAO is biosynthesized from trimethylamine, which is derived from choline.[3]
Trimethylaminuria
Trimethylaminuria is a rare defect in the production of the enzyme flavin containing monooxygenase 3 (FMO3).[4][5] Those suffering from trimethylaminuria are unable to convert choline-derived trimethylamine into trimethylamine oxide. Trimethylamine then accumulates and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Laboratory applications
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.[6]
In the organometallic chemistry reaction of nucleophilic abstraction, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
- M(CO)n + Me3NO + L → M(CO)n-1L + Me3N + CO2
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.[2]
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the corresponding aldehyde.[7]
Microbiotic associations
The order Clostridiales, the genus Ruminococcus, and the taxon Lachnospiraceae are positively associated with TMA and TMAO levels.[8] In contrast, proportions of S24-7, an abundant family from Bacteroidetes, are inversely associated with TMA and TMAO levels.[8]
Health issues
Studies published in 2013 indicate that high levels of TMAO in the blood are associated with an increased risk of major adverse cardiovascular events.[9] The concentration of TMAO in the blood increases after consuming foods containing carnitine[10] or lecithin[9] if the bacteria that convert those substances to TMAO are present in the gut.[11] High concentrations of carnitine are found in red meat, some energy drinks, and some dietary supplements; lecithin is found in soy, eggs,[11] as an ingredient in processed food and is sold as a dietary supplement. Some types of normal gut bacteria (e.g. species of Acinetobacter) in the human microbiome convert dietary carnitine to TMAO. TMAO alters cholesterol metabolism in the intestines, in the liver, and in artery wall. In the presence of TMAO, there is increased deposition of cholesterol in, and decreased removal of cholesterol from, peripheral cells such as those in the artery wall.[12]
The link between cardiovascular diseases and TMAO is disputed by other researchers.[13] Clouatre et al. argue that choline sources and dietary L-carnitine do not contribute to a significant elevation of blood TMAO, and the main TMAO source in the diet is fish.[14]
Another source of TMAO is dietary phosphatidylcholine, again by way of bacterial action in the gut. Phosphatidyl choline is present at high concentration in egg yolks and some meats.
It has been suggested that TMAO may be involved in the regulation of arterial blood pressure and etiology of hypertension[15] and thrombosis (blood clots) in atherosclerotic disease.[16]
Inhibition
Vegan and vegetarian diets appear to select against gut flora that metabolize carnitine (in favor of other gut flora more coordinated with their food supply). This apparent difference in their microbiome is associated with substantially reduced gut bacteria capable of converting carnitine to trimethylamine, which is later metabolized in the liver to TMAO.[10]
3,3-Dimethyl-1-butanol (DMB), a structural analog of choline, inhibits microbial TMA formation in mice and in human feces, thereby reducing plasma TMAO levels after choline or carnitine supplementation.[8] It is found in some balsamic vinegars, red wines, and some cold-pressed extra virgin olive oils and grape seed oils.[8]
References
- ↑ Yancey, P. (2005). "Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses". J. Exp. Biol. 208 (15): 2819–2830. doi:10.1242/jeb.01730. PMID 16043587.
- 1 2 A. J. Pearson "Trimethylamine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2001: New York. doi:10.1002/047084289X.rt268
- ↑ Baker, J.R.; Chaykin, S. (1 April 1962). "The biosynthesis of trimethylamine-N-oxide". J. Biol. Chem. 237 (4): 1309–13. PMID 13864146.
- ↑ Treacy, E.P.; Akerman, BR; et al. (1998). "Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication". Human Molecular Genetics. 7 (5): 839–45. doi:10.1093/hmg/7.5.839. PMID 9536088.
- ↑ Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E (1999). "Mild trimethylaminuria caused by common variants in FMO3 gene". Lancet. 354 (9181): 834–5. doi:10.1016/S0140-6736(99)80019-1. PMID 10485731.
- ↑ Zou, Q.; et al. (2002). "The Molecular Mechanism of Stabilization of Proteins by TMAO and Its Ability to Counteract the Effects of Urea". J. Am. Chem. Soc. 124 (7): 1192–1202. doi:10.1021/ja004206b. PMID 11841287.
- ↑ Volker Franzen (1973). "Octanal". Org. Synth.; Coll. Vol., 5, p. 872
- 1 2 3 4 Wang, Zeneng; Roberts, Adam B.; Buffa, Jennifer A.; Levison, Bruce S.; Zhu, Weifei; Org, Elin; Gu, Xiaodong; Huang, Ying; Zamanian-Daryoush, Maryam; Culley, Miranda K.; DiDonato, Anthony J.; Fu, Xiaoming; Hazen, Jennie E.; Krajcik, Daniel; DiDonato, Joseph A.; Lusis, Aldons J.; Hazen, Stanley L. (December 2015). "Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis". Cell. 163 (7): 1585–1595. doi:10.1016/j.cell.2015.11.055.
• A structural analog of choline, 3,3-dimethyl-1-butanol (DMB), is shown to non-lethally inhibit TMA formation from cultured microbes, to inhibit distinct microbial TMA lyases, and to both inhibit TMA production from physiologic polymicrobial cultures (e.g., intestinal contents, human feces) and reduce TMAO levels in mice fed a high-choline or L-carnitine diet.
• DMB was detected in some balsamic vinegars, in red wines, and in some cold-pressed extra virgin olive oils and grape seed oils - 1 2 Tang, W.H. Wilson; Zeneng Wang; Bruce S. Levison; Robert A. Koeth; Earl B. Britt; Xiaoming Fu; Yuping Wu; Stanley L. Hazen (April 25, 2013). "Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk". The New England Journal of Medicine. 368 (17): 1575–1584. doi:10.1056/NEJMoa1109400. PMC 3701945
 . PMID 23614584. Retrieved April 26, 2013.
. PMID 23614584. Retrieved April 26, 2013. The production of TMAO from dietary phosphatidylcholine is dependent on metabolism by the intestinal microbiota. Increased TMAO levels are associated with an increased risk of incident major adverse cardiovascular events
- 1 2 Koeth, Robert A; Wang, Zeneng; Levison, Bruce S; Buffa, Jennifer A; Org, Elin; Sheehy, Brendan T; Britt, Earl B; Fu, Xiaoming; Wu, Yuping; Li, Lin; Smith, Jonathan D; DiDonato, Joseph A; Chen, Jun; Li, Hongzhe; Wu, Gary D; Lewis, James D; Warrier, Manya; Brown, J Mark; Krauss, Ronald M; Tang, W H Wilson; Bushman, Frederic D; Lusis, Aldons J; Hazen, Stanley L (April 7, 2013). "Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis". Nature Medicine. 19 (5): 576–85. doi:10.1038/nm.3145. PMC 3650111
 . PMID 23563705. Retrieved April 26, 2013.
. PMID 23563705. Retrieved April 26, 2013. - 1 2 Gina Kolata (April 24, 2013). "Eggs, Too, May Provoke Bacteria to Raise Heart Risk". The New York Times. Retrieved April 25, 2013.
- ↑ Hazen, Stanley. "New Research On Red Meat And Heart Disease". The Diane Rehm Show (Transcript). WAMU 88.5 American University Radio. Retrieved 10 April 2013.
- ↑ Collins, Heidi L.; Drazul-Schrader, Denise; Sulpizio, Anthony C.; Koster, Paul D.; Williamson, Yuping; Adelman, Steven J.; Owen, Kevin; Sanli, Toran; Bellamine, Aouatef (2016). "L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP". Atherosclerosis. 244: 29–37. doi:10.1016/j.atherosclerosis.2015.10.108. ISSN 0021-9150.
- ↑ Johri, A.M.; Heyland, D.K.; Hétu, M.-F.; Crawford, B.; Spence, J.D. (2014). "Carnitine therapy for the treatment of metabolic syndrome and cardiovascular disease: Evidence and controversies". Nutrition, Metabolism and Cardiovascular Diseases. 24 (8): 808–814. doi:10.1016/j.numecd.2014.03.007. ISSN 0939-4753.
- ↑ http://www.onlinecjc.ca/article/S0828-282X%2814%2901363-4/abstract
- ↑ Tilg, Herbert (2016-06-22). "A Gut Feeling about Thrombosis". New England Journal of Medicine. 374 (25): 2494–2496. doi:10.1056/nejmcibr1604458.