Trichoderma
| Trichoderma | |
|---|---|
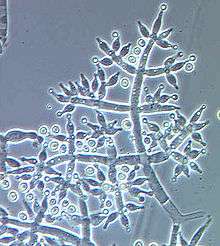 | |
| Trichoderma harzianum | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Subdivision: | Pezizomycotina |
| Class: | Sordariomycetes |
| Order: | Hypocreales |
| Family: | Hypocreaceae |
| Genus: | Trichoderma Persoon |
| Species | |
|
see text | |
Trichoderma is a genus of fungi that is present in all soils, where they are the most prevalent culturable fungi. Many species in this genus can be characterized as opportunistic avirulent plant symbionts.[1] This refers to the ability of several Trichoderma species to form mutualistic endophytic relationships with several plant species.[2] The genomes of several Trichoderma species have been sequenced and are publicly available from the JGI.[3]
Taxonomy
The genus was described by Christiaan Hendrik Persoon in 1794, but the taxonomy has remained difficult to resolve. For a long time it was considered to consist of only one species, Trichoderma viride, named for producing green mold.[4]
Subdivision
The genus was divided into five sections in 1991 by Bissett, partly based on the aggregate species described by Rifai:[5]
- Pachybasium (20 species)
- Longibrachiatum (10 species)
- Trichoderma
- Saturnisporum (2 species)
- Hypocreanum
With the advent of molecular markers from 1995 onwards, Bissett's scheme was largely confirmed but Saturnisporum was merged with Longibrachiatum. While Longibrachiatum and Hypocreanum appeared monophyletic, Pachybasium was determined to be paraphyletic, many of its species clustering with Trichoderma. Druzhina and Kubicek (2005) confirmed the genus as circumscribed was holomorphic. They identified 88 species which they demonstrated could be assigned to two major clades.[4] Consequently, the formal description of sections has been largely replaced by informal descriptions of clades, such as the Aureoviride clade or the Gelatinosum clade.
Species
The belief that Trichoderma was monotypic persisted until the work of Rifai in 1969, who recognised nine species.[6] Currently there are 89 accepted species in the Trichoderma genus. Hypocrea are teleomorphs of Trichoderma which themselves have Hypocrea as anamorphs.[6]
Characteristics
Cultures are typically fast growing at 25–30 °C, but some species of Trichoderma will grow at 45 °C. Colonies are transparent at first on media such as cornmeal dextrose agar (CMD) or white on richer media such as potato dextrose agar (PDA). Mycelium are not typically obvious on CMD, conidia typically form within one week in compact or loose tufts in shades of green or yellow or less frequently white. A yellow pigment may be secreted into the agar, especially on PDA. Some species produce a characteristic sweet or 'coconut' odor.
Conidiophores are highly branched and thus difficult to define or measure, loosely or compactly tufted, often formed in distinct concentric rings or borne along the scant aerial hyphae. Main branches of the conidiophores produce lateral side branches that may be paired or not, the longest branches distant from the tip and often phialides arising directly from the main axis near the tip. The branches may rebranch, with the secondary branches often paired and longest secondary branches being closest to the main axis. All primary and secondary branches arise at or near 90° with respect to the main axis. The typical Trichoderma conidiophore, with paired branches assumes a pyramidal aspect. Typically the conidiophore terminates in one or a few phialides. In some species (e.g. T. polysporum) the main branches are terminated by long, simple or branched, hooked, straight or sinuous, septate, thin-walled, sterile or terminally fertile elongations. The main axis may be the same width as the base of the phialide or it may be much wider.
Phialides are typically enlarged in the middle but may be cylindrical or nearly subglobose. Phialides may be held in whorls, at an angle of 90° with respect to other members of the whorl, or they may be variously penicillate (gliocladium-like). Phialides may be densely clustered on wide main axis (e.g. T. polysporum, T. hamatum) or they may be solitary (e.g. T. longibrachiatum).
Conidia typically appear dry but in some species they may be held in drops of clear green or yellow liquid (e.g. T. virens, T. flavofuscum). Conidia of most species are ellipsoidal, 3–5 x 2–4 µm (L/W = > 1.3); globose conidia (L/W < 1.3) are rare. Conidia are typically smooth but tuberculate to finely warted conidia are known in a few species.
Synanamorphs are formed by some species that also have typical Trichoderma pustules. Synanamorphs are recognized by their solitary conidiophores that are verticillately branched and that bear conidia in a drop of clear green liquid at the tip of each phialide.
Chlamydospores may be produced by all species, but not all species produce chlamydospores on CMD at 20 °C within 10 days. Chlamydospores are typically unicellular subglobose and terminate short hyphae; they may also be formed within hyphal cells. Chlamydospores of some species are multicellular (e.g. T. stromaticum).
Trichoderma genomes appear to be in the 30–40 Mb range, with approximately 12,000 genes being identifiable.
Teleomorph
Teleomorphs of Trichoderma are species of the ascomycete genus Hypocrea. These are characterized by the formation of fleshy, stromata in shades of light or dark brown, yellow or orange. Typically the stroma is discoidal to pulvinate and limited in extent but stromata of some species are effused, sometimes covering extensive areas. Stromata of some species (Podostroma) are clavate or turbinate. Perithecia are completely immersed. Ascospores are bicellular but disarticulate at the septum early in development into 16 part-ascospores so that the ascus appears to contain 16 ascospores. Ascospores are hyaline or green and typically spinulose. More than 200 species of Hypocrea have been described but few have been grown in pure culture and even fewer have been described in modern terms.
Occurrence

Trichoderma species are frequently isolated from forest or agricultural soils at all latitudes. Hypocrea species are most frequently found on bark or on decorticated wood but many species grow on bracket fungi (e.g. H. pulvinata), Exidia (H. sulphurea) or bird's nest fungi (H. latizonata) or agarics (H. avellanea).
Biocontrol agent
Several strains of Trichoderma have been developed as biocontrol agents against fungal diseases of plants.[7] The various mechanisms include antibiosis, parasitism, inducing host-plant resistance, and competition. Most biocontrol agents are from the species T. harzianum, T. viride and T. hamatum. The biocontrol agent generally grows in its natural habitat on the root surface, and so affects root disease in particular, but can also be effective against foliar diseases.
Causal agent of disease
T. aggressivum (formerly T. harzianum biotype 4) is the causal agent of green mold, a disease of cultivated button mushrooms.[8] .[9] Trichoderma viride is the causal agent of green mold rot of onion (evidence?). But, Dieback of Pinus nigra seedlings caused by a strain of Trichoderma viride is known.[10]
Toxic house mold
The common house mold, Trichoderma longibrachiatum, produces small toxic peptides containing amino acids not found in common proteins, like alpha-aminoisobutyric acid, called trilongins (up to 10% w/w). Their toxicity is due to absorption into cells and production of nano-channels that obstruct vital ion channels that ferry potassium and sodium ions across the cell membrane. This affects in the cells action potential profile, as seen in cardiomyocytes, pneumocytes and neurons leading to conduction defects. Trilongins are highly resistant to heat and antimicrobials making primary prevention the only management option.[11][12][13]
Medical uses
Cyclosporine A (CsA), a calcineurin inhibitor produced by the fungi Trichoderma polysporum,[14] Tolypocladium inflatum and Cylindrocarpon lucidum, is an immunosuppressant prescribed in organ transplants to prevent rejection.[15]
Industrial use
Trichoderma, being a saprophyte adapted to thrive in diverse situations, produces a wide array of enzymes. By selecting strains that produce a particular kind of enzyme, and culturing these in suspension, industrial quantities of enzyme can be produced.
- Trichoderma reesei is used to produce cellulase and hemicellulase [16]
- Trichoderma longibrachiatum is used to produce xylanase [17]
- Trichoderma harzianum is used to produce chitinase.[18]
See also
References
- ↑ Harman, G.E., Howell, C.R., Viterbo, A., Chet, I., Lorito, M. (2004). "Trichoderma species—opportunistic avirulent plant symbionts". Nature Reviews Microbiology. 2 (1): 43–56. doi:10.1038/nrmicro797. PMID 15035008.
- ↑ Bae, H; Roberts, DP; Lim, HS; Strem, MD; Park, SC; Ryu, CM; Melnick, RL; Bailey, BA (2011). "Endophytic Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms". Mol. Plant Microbe Interact. 24: 336–51. doi:10.1094/MPMI-09-10-0221. PMID 21091159.
- ↑ http://genome.jgi-psf.org/Triat1/Triat1.home.html
- 1 2 Druzhinina, Irina; Kubicek, Christian P. (February 2005). "Species concepts and biodiversity in Trichoderma and Hypocrea : from aggregate species to species clusters?". Journal of Zhejiang University SCIENCE. 6B (2): 100–112. doi:10.1631/jzus.2005.B0100. PMC 1389624
 . PMID 15633245.
. PMID 15633245. - ↑ Bissett, John (November 1991). "A revision of the genus Trichoderma. III. Section Pachybasium". Canadian Journal of Botany. 69 (11): 2373–2417. doi:10.1139/b91-298. Retrieved 7 December 2014.
- 1 2 Samuels, Gary J. (2006). "Trichoderma: Systematics, the Sexual State, and Ecology". Phytopathology. 96 (2): 195–206. doi:10.1094/PHYTO-96-0195. ISSN 0031-949X. PMID 18943925.
- ↑ Harman, G.E. (2006). "Overview of mechanisms and uses of Trichoderma spp.". Phytopathology. 96 (2): 190–194. doi:10.1094/PHYTO-96-0190. PMID 18943924.
- ↑ Beyer, W.M, Wuest, P.J., Anderson, M.G. "Green mold of Mushrooms". Pennsylvania State University. Retrieved 2007-08-02. Pennsylvania State University extension bulletin
- ↑ Samuels, G.J., Dodd, S.L., Gams, W., Castlebury, L.A., Petrini, O. (2002). "Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus". Mycologia. 94 (1): 146–170. doi:10.2307/3761854. JSTOR 3761854.
- ↑ Li Destri Nicosia, M.G., Mosca, S., Mercurio, R., Schena, L. (2015). "Dieback of Pinus nigra Seedlings Caused by a Strain of Trichoderma viride". Plant Disease. 99 (1): 44–49. doi:10.1094/PDIS-04-14-0433-RE.
- ↑ Reason Discovered for the Toxicity of Indoor Mould – ScienceDaily (Oct. 12, 2012) : http://www.sciencedaily.com/releases/2012/10/121012074655.htm
- ↑ "20-Residue and 11-residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming Na + /K + -permeable channels and adverse action towards mammalian cells". FEBS Journal. 279: 4172–4190. doi:10.1111/febs.12010.
- ↑ “Trilongins” Offer Insight into Mold Toxicity Environmental health perspectives 2/2013.
- ↑ Dreyfuss, M; Härri, E; Hofmann, H; Kobel, H; Pache, W; Tscherter, H (1976). "Cyclosporin A and C: new metabolites from Trichoderma polysporum (Link ex Pers.) Rifai". European Journal of Applied Microbiology. 3: 125–133. doi:10.1007/bf00928431.
- ↑ Authors; Chong, FW; Chakravarthi, S; Nagaraja, HS; Thanikachalam, PM; Lee, N (Jun 2009). "Expression of transforming growth factor-beta and determination of apoptotic index in histopathological sections for assessment of the effects of Apigenin (4', 5', 7'- Trihydroxyflavone) on Cyclosporine A induced renal damage". Malays J Pathol. 31 (1): 35–43.
- ↑ http://genome.jgi-psf.org/Trire2/Trire2.home.html
- ↑ Azin, M., Moravej, R., Zareh, D. (2007). "Self-directing optimization of parameters for extracellular chitinase production by Trichoderma harzianum in batch mode". Process Biochemistry. 34 (6–7): 563–566. doi:10.1016/S0032-9592(98)00128-9.
- ↑ Felse, P.A, Panda, T. (1999). "Production of xylanase by Trichoderma longibrachiatum on a mixture of wheat bran and wheat straw: Optimization of culture condition by Taguchi method". Enzyme and Microbial Technology. 40 (4): 801–805. doi:10.1016/j.enzmictec.2006.06.013.
Bibliography
- Rifai, M. A. 1969. A revision of the genus Trichoderma. Mycol. Pap. 116:1-56.
- Druzhinina, Irina; Kubicek, Christian P. (February 2005). "Species concepts and biodiversity in Trichoderma and Hypocrea : from aggregate species to species clusters?". Journal of Zhejiang University SCIENCE. 6B (2): 100–112. doi:10.1631/jzus.2005.B0100. PMC 1389624
 . PMID 15633245.
. PMID 15633245.
![]() This article incorporates public domain material from websites or documents of the United States Department of Agriculture.
This article incorporates public domain material from websites or documents of the United States Department of Agriculture.
External links
| Wikispecies has information related to: Trichoderma |
- Samuels, G.J., Chaverri, P., Farr, D.F., & McCray, E.B. (n.d.) Trichoderma Online, Systematic Botany & Mycology Laboratory, ARS, USDA. Retrieved August 3, 2007 This site presents descriptions and over 500 images for the 32 species of Trichoderma that represent a majority of the taxa found in temperate regions
- International Subcommission on Trichoderma and Hypocrea Taxonomy site.