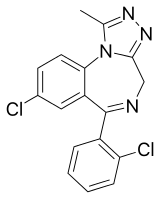
Triazolam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Halcion |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684004 |
| Pregnancy category |
|
| Dependence liability | High |
| Routes of administration | Oral |
| ATC code | N05CD05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 44% (oral route), 53% (sublingual), 98% (intranasal) [[1]] |
| Metabolism | Hepatic |
| Biological half-life | 1.5–5.5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
28911-01-5 |
| PubChem (CID) | 5556 |
| IUPHAR/BPS | 7313 |
| DrugBank |
DB00897 |
| ChemSpider |
5355 |
| UNII |
1HM943223R |
| KEGG |
D00387 |
| ChEBI |
CHEBI:9674 |
| ChEMBL |
CHEMBL646 |
| ECHA InfoCard | 100.044.811 |
| Chemical and physical data | |
| Formula | C17H12Cl2N4 |
| Molar mass | 343.2 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Triazolam (original brand name Halcion) is a central nervous system (CNS) depressant in the benzodiazepine class.[2] It possesses pharmacological properties similar to that of other benzodiazepines, but it is generally only used as a sedative to treat severe insomnia.[3] In addition to the hypnotic properties triazolam possesses, amnesic, anxiolytic, sedative, anticonvulsant and muscle relaxant properties are also present.[4] Due to its short half-life, triazolam is not effective for patients that suffer from frequent awakenings or early wakening.[5]
Triazolam was initially patented in 1970 and went on sale in the United States in 1982.[6]
Medical uses
Triazolam is usually used for short-term treatment of acute insomnia and circadian rhythm sleep disorders, including jet lag. It is an ideal benzodiazepine for this use because its fast onset of action and short half-life. It puts one to sleep for not more than 1.5 hours (approximately 1–2 hours), allowing its user to avoid morning drowsiness. Triazolam is also sometimes used as an adjuvant in medical procedures requiring anesthesia[3] or to reduce anxiety during brief events like MRI scans and non-surgical dental procedures. Triazolam is ineffective in maintaining sleep however, due to its short half-life with quazepam showing superiority.[7]
Triazolam is frequently prescribed as a sleep aid for passengers travelling on short to medium duration flights. If this use is contemplated, it is especially important to avoid the consumption of alcoholic beverages, and to do a ground based "trial" of the medication to ensure that the side effects and potency of this medication are understood by the user prior to using it in a relatively more public environment (as disinhibition can be a common side effect, with potentially severe consequences).
Side effects
Adverse drug reactions associated with the use of triazolam include:
- Relatively common (>1% of patients): somnolence, dizziness, feeling of lightness, coordination problems.
- Less common (0.9% to 0.5% of patients): euphoria, tachycardia, tiredness, confusional states/memory impairment, cramps/pain, depression, visual disturbances.
- Rare (<0.5% of patients): constipation, taste alteration, diarrhea, dry mouth, dermatitis/allergy, dreams/nightmares, insomnia, parasthesia, tinnitus, dysesthesia, weakness, congestion.[8]
Triazolam, although a short-acting benzodiazepine, may still cause residual impairment into the next day, especially the next morning. A meta-analysis demonstrated that residual "hangover" effects after nighttime administration of triazolam such as sleepiness, impaired psychomotor, and cognitive functions may persist into the next day, which may impair the ability of users to drive safely and increase risks of falls and hip fractures.[9] Confusion and amnesia has been reported.[10]
Tolerance, dependence, and withdrawal
A review of the literature found that long-term use of benzodiazepines, including triazolam, is associated with drug tolerance, drug dependence, rebound insomnia, and CNS related adverse effects. It recommended that benzodiazepine hypnotics are used at their lowest possible dose and for a short period of time. Non-pharmacological treatment options however, were found to have sustained improvements in sleep quality.[11] A worsening of insomnia (rebound insomnia) compared to baseline may occur after discontinuation of triazolam even after short-term single-nightly-dose therapy.[12]
Other withdrawal symptoms can range from mild unpleasant feelings to a major withdrawal syndrome, including stomach cramps, vomiting, muscle cramps, sweating, tremor, and, in rare cases, convulsions.[13]
Contraindications
Benzodiazepines require special precaution if used in the elderly, during pregnancy, in children, alcoholics, or other drug-dependent individuals and individuals with comorbid psychiatric disorders.[14] Triazolam belongs to the Pregnancy Category X of the FDA.[15] This means that it is known to have the potential to cause birth defects.
Elderly
Triazolam, similar to other benzodiazepines and nonbenzodiazepines, causes impairments in body balance and standing steadiness in individuals who wake up at night or the next morning. Falls and hip fractures are frequently reported. The combination with alcohol increases these impairments. Partial, but incomplete tolerance develops to these impairments.[16] There can be daytime withdrawal effects.[17]
An extensive review of the medical literature regarding the management of insomnia and the elderly found that there is considerable evidence of the effectiveness and durability of non-drug treatments for insomnia in adults of all ages and that these interventions are underutilized. Compared with the benzodiazepines including triazolam, the nonbenzodiazepine sedative-hypnotics appeared to offer few, if any, significant clinical advantages in efficacy or tolerability in elderly persons. It was found that newer agents with novel mechanisms of action and improved safety profiles, such as the melatonin agonists, hold promise for the management of chronic insomnia in elderly people. Long-term use of sedative-hypnotics for insomnia lacks an evidence base and has traditionally been discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment, anterograde amnesia, daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls.[17] One study found no evidence of sustained hypnotic efficacy throughout the 9 weeks of treatment for triazolam.[17]
In addition, the effectiveness and safety of long-term use of these agents remain to be determined. It was concluded that more research is needed to evaluate the long-term effects of treatment and the most appropriate management strategy for elderly persons with chronic insomnia.[18]
Interactions
Ketoconazole and Itraconazole have a profound effect on the pharmacokinetics of triazolam, leading to greatly enhanced effects.[19] Anxiety, tremor and depression have been documented in a case report following administration of nitrazepam and triazolam. Following administration of erythromycin, repetitive hallucinations and abnormal bodily sensations developed. The patient had, however, acute pneumonia and renal failure. Co-administration of benzodiazepine drugs at therapeutic doses with erythromycin may cause serious psychotic symptoms, especially in those with other physical complications.[20] Caffeine reduces the effectiveness of triazolam.[21] Other important interactions include cimetidine, diltiazem, erythromycin, fluconazole, grapefruit juice, isoniazid, itraconazole, ketoconazole, nefazodone, rifampicin, ritonavir, and troleandomycin.[22][23] Triazolam should not be administered to patients on Atripla.
Overdose
Symptoms of an overdose[3] include
- Coma
- Hypoventilation (respiratory depression)
- Somnolence (drowsiness)
- Slurred speech
- Seizures have been reported.[8]
Death can occur from triazolam overdose but is more likely to occur in combination with other depressant drugs such as opioids, alcohol, or tricyclic antidepressants.[24]
Pharmacology
The pharmacological effects of triazolam are similar to those of most other benzodiazepines. Triazolam does not generate active metabolites.[3] Triazolam is a short acting benzodiazepine, is lipophilic, and is metabolised hepatically via oxidative pathways. The main pharmacological effects of triazolam are the enhancement of the neurotransmitter GABA at the GABAA receptor.[25] The half-life of triazolam is only 2 hours making it a very short acting benzodiazepine drug.[26] Triazolam has anticonvulsant effects on brain function.[27]
History
Its use at low doses has been deemed acceptable by the American Food and Drug Administration (FDA) and several other countries.[3]
Society and culture
Recreational use
Triazolam is a drug that is used non-medically: recreational use wherein the drug is taken to achieve a high or continued long-term dosing against medical advice.[28]
Legal status
Triazolam is a Schedule IV drug under the Convention on Psychotropic Substances[29] and the US Controlled Substances Act.
Brandnames
Marketed in English-speaking countries under the brand names Apo-Triazo, Halcion, Hypam, and Trilam. Other (designer) names include 2'-chloroxanax, chloroxanax, 2'-chloro-alprazolam, triclazolam, and chlorotriazolam.
References
- ↑ "Intranasal absorption of flurazepam, midazolam, and triazolam in dogs". J Pharm Sci. 80: 1125–9. 1991. doi:10.1002/jps.2600801207. PMID 1815070.
- ↑ "Benzodiazepine Names". non-benzodiazepines.org.uk. Retrieved 2008-12-29.
- 1 2 3 4 5 Wishart, David (2006). "Triazolam". DrugBank. Retrieved 2006-03-23.
- ↑ Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Curr. Drug Metab. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614.
- ↑ Rickels K. (1986). "The clinical use of hypnotics: indications for use and the need for a variety of hypnotics". Acta Psychiatrica Scandinavica Suppl. 74 (S332): 132–41. doi:10.1111/j.1600-0447.1986.tb08990.x. PMID 2883820.
- ↑ Shorter, Edward (2005). "B". A Historical Dictionary of Psychiatry. Oxford University Press. ISBN 9780190292010.
- ↑ Mauri MC, Gianetti S, Pugnetti L, Altamura AC (1993). "Quazepam versus triazolam in patients with sleep disorders: a double-blind study". Int J Clin Pharmacol Res. 13 (3): 173–7. PMID 7901174.
- 1 2 http://www.pfizer.com/files/products/uspi_halcion.pdf
- ↑ Vermeeren A. (2004). "Residual effects of hypnotics: epidemiology and clinical implications". CNS Drugs. 18 (5): 297–328. doi:10.2165/00023210-200418050-00003. PMID 15089115.
- ↑ Lieberherr, S; Scollo-Lavizzari, G; Battegay, R (1991). "Confusional states following administration of short-acting benzodiazepines (midazolam/triazolam)". Schweizerische Rundschau fur Medizin Praxis = Revue suisse de médecine Praxis. 80 (24): 673–5. PMID 2068441.
- ↑ Kirkwood CK (1999). "Management of insomnia". J Am Pharm Assoc (Wash). 39 (5): 688–96; quiz 713–4. PMID 10533351.
- ↑ Kales A; Scharf MB; Kales JD; Soldatos CR. (1979-04-20). "Rebound insomnia. A potential hazard following withdrawal of certain benzodiazepines". JAMA : the Journal of the American Medical Association. 241 (16): 1692–5. doi:10.1001/jama.241.16.1692. PMID 430730.
- ↑ "www.accessdata.fda.gov" (PDF).
- ↑ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM.; Eschalier, A. (Nov 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr. 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ↑ http://www.fda.gov/medwatch/SAFETY/2003/03Jun_PI/Halcion_PI.pdf
- ↑ Mets, MA.; Volkerts, ER.; Olivier, B.; Verster, JC. (Feb 2010). "Effect of hypnotic drugs on body balance and standing steadiness". Sleep Med Rev. 14 (4): 259–67. doi:10.1016/j.smrv.2009.10.008. PMID 20171127.
- 1 2 3 Bayer, A.J.; Bayer EM; Pathy MSJ; Stoker MJ. (1986). "A Double-Blind Controlled Study of Chlormethiazole and Triazolam in the Elderly.". Acta Psychiatrica Scandinavica. 73 (suppl 329): 104–111. doi:10.1111/j.1600-0447.1986.tb10544.x. PMID 3529832.
- ↑ Bain KT (June 2006). "Management of chronic insomnia in elderly persons". Am J Geriatr Pharmacother. 4 (2): 168–92. doi:10.1016/j.amjopharm.2006.06.006. PMID 16860264.
- ↑ Varhe A, Olkkola KT, Neuvonen PJ (December 1994). "Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazole". Clin. Pharmacol. Ther. 56 (6 Pt 1): 601–7. doi:10.1038/clpt.1994.184. PMID 7995001.
- ↑ Tokinaga N; Kondo T; Kaneko S; Otani K; Mihara K; Morita S. (December 1996). "Hallucinations after a therapeutic dose of benzodiazepine hypnotics with co-administration of erythromycin". Psychiatry and clinical neurosciences. 50 (6): 337–9. doi:10.1111/j.1440-1819.1996.tb00577.x. PMID 9014234.
- ↑ Mattila, Me; Mattila, Mj; Nuotto, E (April 1992). "Caffeine moderately antagonizes the effects of triazolam and zopiclone on the psychomotor performance of healthy subjects". Pharmacology & toxicology. 70 (4): 286–9. doi:10.1111/j.1600-0773.1992.tb00473.x. ISSN 0901-9928. PMID 1351673.
- ↑ Wang JS, DeVane CL (2003). "Pharmacokinetics and drug interactions of the sedative hypnotics" (PDF). Psychopharmacol Bull. 37 (1): 10–29. doi:10.1007/BF01990373. PMID 14561946.
- ↑ Arayne, MS.; Sultana, N.; Bibi, Z. (Oct 2005). "Grape fruit juice-drug interactions". Pak J Pharm Sci. 18 (4): 45–57. PMID 16380358.
- ↑ Kudo K, Imamura T, Jitsufuchi N, Zhang XX, Tokunaga H, Nagata T (April 1997). "Death attributed to the toxic interaction of triazolam, amitriptyline and other psychotropic drugs". Forensic Sci. Int. 86 (1–2): 35–41. doi:10.1016/S0379-0738(97)02110-5. PMID 9153780.
- ↑ Oelschläger H. (1989-07-04). "Chemical and pharmacologic aspects of benzodiazepines". Schweiz Rundsch Med Prax. 78 (27–28): 766–72. PMID 2570451.
- ↑ Professor heather Ashton (April 2007). "BENZODIAZEPINE EQUIVALENCY TABLE". Retrieved September 23, 2007.
- ↑ Chweh AY; Swinyard EA; Wolf HH; Kupferberg HJ (February 25, 1985). "Effect of GABA agonists on the neurotoxicity and anticonvulsant activity of benzodiazepines". Life Sci. 36 (8): 737–44. doi:10.1016/0024-3205(85)90193-6. PMID 2983169.
- ↑ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry. 66 Suppl 9: 31–41. PMID 16336040.
- ↑ "List of psychotropic substances under international control" (PDF). Green list. International Narcotics Control Board. YEAR. Retrieved 2006-03-23. Check date values in:
|date=(help)
External links
- PubPK - Triazolam Pharmacokinetics
- Medlineplus.org - Triazolam
- Rx-List.com - Triazolam
- Inchem.org - Triazolam
- MentalHealth.com - Triazolam
- Halcion controversy - Newsweek August 19, 1991 - Sweet Dreams or Nightmare?
- http://www.sussex.ac.uk/sociology/documents/ssm02.pdf