Prelog strain

In organic chemistry, transannular strain (also called Prelog strain after Nobel Prize–winning chemist Vladimir Prelog) is the unfavorable interactions of ring substituents on non-adjacent carbons. These interactions, called transannular interactions, arise from a lack of space in the interior of the ring, which forces substituents into conflict with one another. In medium-sized cycloalkanes, which have between 8 and 11 carbons constituting the ring, transannular strain is a major source of the overall strain, to which there is also contribution from large-angle strain and Pitzer strain.[1][2] In larger rings, transannular strain drops off until the ring is sufficiently large that it can adopt conformations devoid of any negative interactions.[1][3]
Transannular strain can also be demonstrated in other cyclo-organic molecules, such as lactones, lactams, ethers, cycloalkenes, and cycloalkynes. These compounds are not without significance, since they are particularly useful in the study of transannular strain. Furthermore, transannular interactions are not relegated to only conflicts between hydrogen atoms, but can also arise from larger, more complicated substituents interacting across a ring.
Thermodynamics

By definition, strain implies discomfiture, so it should follow that molecules with large amounts of transannular strain should have higher energies than those without. Cyclohexane, for the most part, is without strain and is therefore quite stable and low in energy. Rings smaller than cyclohexane, like cyclopropane and cyclobutane, have significant tension caused by small-angle strain, but there is no transannular strain. While there is no small-angle strain present in medium-sized rings, there does exist something called large-angle strain. Large-angle strain is used by rings with more than nine members to relieve some of the distress caused by transannular strain.[1][3]
As the plot to the left indicates, the relative energies of cycloalkanes increases as the size of the ring increases, with a peak at cyclononane (with nine members in its ring.) At this point, the flexibility of the rings increases with increasing size; this allows for conformations that can significantly mitigate transannular interactions. Some of this strain is certainly due to Pitzer strain and large-angle strain, but a large amount is still caused by transannular strain.[1]
Kinetics

Rates of reaction can be affected by the size of rings. Essentially each reaction should be studied on a case by case basis but some general trends have been seen. Rings with transannular strain have faster SN1, SN2, and free radical reactions compared to most smaller and normal sized rings. Five membered rings show an exception to this trend. On the other hand, some nucleophilic addition reactions involving addition to a carbonyl group in general show the opposite trend. Smaller and normal rings, with five membered rings being the anomaly, have faster reaction rates while those with transannular strain are slower.[4]

| n | k1 h−1 at 25 °C | Relative rate |
|---|---|---|
| 4 | 0.00224 | 0.211 |
| 5 | 1.32 | 124 |
| 6 | 0.0106 | 1.00 |
| 7 | 1.15 | 108 |
| 8 | 3.03 | 286 |
| 9 | 0.465 | 43.9 |
| 10 | 0.188 | 17.7 |
| 11 | 0.127 | 12.0 |
| 13 | 0.0302 | 2.85 |
| 15 | 0.0192 | 1.81 |
| 17 | 0.0201 | 1.90 |
One specific example of a study of rates of reactions for an SN1 reaction is shown on the right. Various sized rings, ranging from four to seventeen members, were used to compare the relative rates and better understand the effect of transannular strain on this reaction. The solvolysis reaction in acetic acid involved the formation of a carbocation as the chloride ion leaves the cyclic molecule. This study fits the general trend seen above that rings with transannular strain show increased reactions rates compared to smaller rings in SN1 reactions.[4]
Examples of transannular strain
Influence on regioselectivity
The regioselectivity of water elimination is highly influenced by ring size. When water is eliminated from cyclic tertiary alcohols by an E1 route, three major products are formed. The semicyclic isomer (so-called because the double bond is shared by a ring atom and an exocyclic atom) and the (E) endocyclic isomer are expected to predominate; the (Z) endocyclic isomer is not expected to be formed until the ring size is large enough to accommodate the awkward angles of the trans configuration. The exact population of each product relative to the others differs considerably depending upon the size of the ring involved. As the ring size increases, the semicyclic isomer decreases rapidly and the (E) endocyclic isomer increases, but after a certain point, the semicyclic isomer begins to increase again. This can be attributed to transannular strain; this strain is significantly reduced in the (E) endocyclic isomer because it has one less substituent in the ring than the semicyclic isomer.[5]
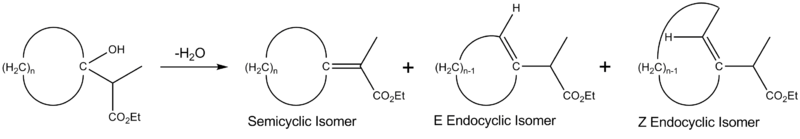

Influence on medium-sized ring synthesis
One of the effects of transannular strain is the difficulty of synthesizing medium-sized rings. Illuminati et al. have studied the kinetics of intramolecular ring closing using the simple nucleophilicsubstitution reaction of ortho-bromoalkoxyphenoxides. Specifically, they studied the ring closing of 5 to 10 carbon cyclic ethers. They found that as the number of carbons increased, so did the enthalpy of activation for the reaction. This indicates that strain within the cyclic transition states is higher if there are more carbons in the ring. Since transannular strain is the largest source of strain in rings this size, the larger enthalpies of activation result in much slower cyclizations due to transannular interactions in the cyclic ethers.[6]
Influence of bridges on transannular strain
Transannular strain can be eliminated by the simple addition of a carbon bridge. E,Z,E,Z,Z-[10]-annulene is quite unstable; while it has the requisite number of π-electrons to be aromatic, they are for the most part isolated. Ultimately, the molecule itself is very difficult to observe. However, by the simple addition of a methylene bridge between the 1 and 6 positions, a stable, flat, aromatic molecule can be made and observed.[7]

References
- 1 2 3 4 Smith and March, March's Advanced Organic Chemistry, John Wiley & Sons Inc., 2007, ISBN 978-0-471-72091-1
- ↑ Raphael, R.A. (1962). "Proceedings of the Chemical Society. March 1962". Proc. Chem. Soc.: 97. doi:10.1039/PS9620000097.
- 1 2 Anslyn and Dougherty, Modern Physical Organic Chemistry, University Science Books, 2006, ISBN 978-1-891389-31-3
- 1 2 3 Goldfarb; Belenkii (1960). "Strain and Reactivity in Monocyclic Systems". Russian Chemical Reviews. 29 (4): 214–235. doi:10.1070/RC1960v029n04ABEH001228.
- ↑ Greve and Imming.; Imming, Peter (1997). "Regio- and Stereoselectivity of Water Elimination as a Function of Ring Size". J. Org. Chem. 62 (23): 8058. doi:10.1021/jo970989g.
- ↑ Illuminati; et al. (1975). "Ring-closure reactions. V. Kinetics of five- to ten-membered ring formation from o-.omega.-bromoalkylphenoxides. Influence of the O-heteroatom". JACS. 97 (17): 4961. doi:10.1021/ja00850a032.
- ↑ Slayden and Liebman. (2001). "The Energetics of Aromatic Hydrocarbons: An Experimental Thermochemical Perspective". Chem. Rev. 101 (5): 1541. doi:10.1021/cr990324+.
External links
- Prelog strain definition: Link
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "transannular strain".