Tröger's base
 | |
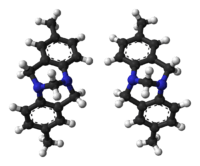 Ball-and-stick models of the two enantiomers of Tröger's base | |
| Names | |
|---|---|
| IUPAC name
2,8-Dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine | |
| Other names
Troeger's base | |
| Identifiers | |
| 529-81-7 (±) 21451-74-1 (+) 14645-24-0 (-) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 170601 |
| ECHA InfoCard | 100.150.499 |
| PubChem | 196981 |
| |
| |
| Properties | |
| C17H18N2 | |
| Molar mass | 250.35 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
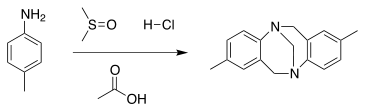
Tröger's base is an organic compound that exhibits chirality due to the presence of two bridgehead stereogenic nitrogen atoms in its structure. The compound was first synthesised in 1887 [1] from p-toluidine and formaldehyde in acid solution by Julius Tröger. It took until 1935 for the elucidation of the molecular structure.[2] Tröger's base can also be prepared with DMSO and hydrochloric acid [3] or hexamethylene tetraamine (HMTA) [4] as formaldehyde replacement .
The reaction mechanism with DMSO as methylene donor for this reaction is similar to that of the Pummerer rearrangement. The interaction of DMSO and hydrochloric acid yields an electrophilic sulfenium ion that reacts with the aromatic amine in an electrophilic addition. Dimethyl sulfide is eliminated and the resulting imine reacts with a second amine. Sulfenium ion addition and elimination is repeated with the second amino group and the imine group reacts in an intramolecular electrophilic aromatic substitution reaction. Imine generation is repeated a third time and the reaction concludes with a second electrophilic substitution to the other aromat.
The molecule can be considered a molecular tweezer because the bicyclic skeleton forces the molecule in a rigid locked conformation with the aromatic rings in proximity.[5] When the methyl groups are replaced by pyridine amide groups a host-guest chemistry interaction can take place between the Tröger's base and an aliphatic dicarboxylic acid.[6] It is found that the cavity dimensions are optimal for inclusion of suberic acid but that with a longer acid sebacic acid or a shorter acid adipic acid the interaction is less favorable.
 Both enantiomers of Tröger's base: (5S,11S)-enantiomer (above) and (5R,11R)-enantiomer
Both enantiomers of Tröger's base: (5S,11S)-enantiomer (above) and (5R,11R)-enantiomer
History
After Tröger's original synthesis in 1887, the structure of the compound was unknown. Because he was not able to give a structure of the new compound, Johannes Wislicenus, the new director of the department, assigned a mediocre grade for Tröger's thesis. Several publications offered possible structures of the compound, but the final, correct structure of the molecule was not elucidated until 1935, 48 years later.
In later years, the structure of Tröger's base was used to demonstrate that not only carbon, but also nitrogen, is capable of forming a chiral center in a molecule. The nitrogen inversion normally leads to rapid equilibriation between the enantiomers of chiral amines, but this can be stopped by conformational strain. In Tröger's base, this inversion is no longer possible, and the nitrogen atoms are chiral centers. The separation of the enantiomers of Tröger's base was first accomplished by Vladimir Prelog in 1944.[7] The use of the column chromatograph with a chiral compound as the stationary phase was a relatively new method, but after this successful demonstration it became a standard procedure.
References
- ↑ Tröger, Julius (1887). "Ueber einige mittelst nascirenden Formaldehydes entstehende Basen". Journal für Praktische Chemie. 36 (1): 225–245. doi:10.1002/prac.18870360123.
- ↑ Spielman, M. A. (1935). "The Structure of Troeger's Base" (PDF). Journal of the American Chemical Society. 57 (3): 583––585. doi:10.1021/ja01306a060.
- ↑ Li, Zhong; Xu, Xiaoyong; Peng, Yanqing; Jiang, Zhaoxing; Ding, Chuanyong; Qian, Xuhong (2005). "An Unusual Synthesis of Tröger's Bases Using DMSO/HCl as Formaldehyde Equivalent". Synthesis (8): 1228–1230. doi:10.1055/s-2005-861868.
- ↑ Masa, Thierry; Pardo, Carmen; Elguero, José (2004). "A shorter synthesis of symmetrical 2,11-dimethyl-bis-Tröger's bases. A new molecular tweezer". Arkivoc. (EM-973K).
- ↑ Pardo, C; Sesmilo, E; Gutiérrez-Puebla, E; Monge, A; Elguero, J; Fruchier, A (2001). "New Chiral Molecular Tweezers with a Bis-Tröger's Base Skeleton". Journal of Organic Chemistry. 66 (5): 1607–1611. doi:10.1021/jo0010882. PMID 11262103.
- ↑ Goswami, S; Ghosh, K; Dasgupta, S (2000). "Troger's Base Molecular Scaffolds in Dicarboxylic Acid Recognition". Journal of Organic Chemistry. 65 (7): 1907–1914. doi:10.1021/jo9909204. PMID 10774008.
- ↑ Prelog, V.; Wieland, P. (1944). "Über die Spaltung der Tröger'schen Base in optische Antipoden, ein Beitrag zur Stereochemie des dreiwertigen Stickstoffs". Helvetica Chimica Acta. 27 (1): 1127–1134. doi:10.1002/hlca.194402701143.