Tocotrienol
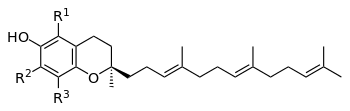
Tocotrienols are members of the vitamin E family.[1] They can be used as a food additive as well as a substitute for Vitamin E (alpha tocopherol equivalent activity) as defined by E-307 . The vitamin E family are essential dietary components as the body can not synthesise sufficient itself. The body contains four tocotrienols (alpha, beta, gamma, delta) and four tocopherols (alpha, beta, gamma, delta) .[2] However these have different antioxidant activities when measured in human plasma [3]
The critical difference between tocotrienols and tocopherols is in that tocopherols have saturated side chains, whereas tocotreinols have unsaturated isoprenoid side chains (farnesyl isoprenoid tails) with three double bonds [4][5]
Tocotrienols have only a single chiral center, which exists at the 2' chromanol ring carbon, at the point where the isoprenoid tail joins the ring. The other two corresponding centers in the phytyl tail of the corresponding tocopherols do not exist due to tocotrienol's unsaturation at these sites. Tocotrienols extracted from natural sources always consist of the dextrorotatory enantiomers only. These naturally occurring, dextrorotatory stereoisomers are generally abbreviated as the "d-" forms, for example, "d-tocotrienol" or "d-alpha-tocotrienol". In theory, the unnatural "l-tocotrienol" (levorotatory) forms of tocotrienols could exist as well, which would have a 2S (rather than 2R) configuration at the molecules' single chiral center. In practice, however, tocotrienols are only produced in the d-form i.e. from natural sources. The synthetic mixed stereoisomer ("dl-tocotrienol") and its acetate are suitable as dietary supplements but although more expensive, the natural d-alpha tocopherol isomer and its acetate are the preferred form due to higher biological activity.
Tocotrienols are compounds naturally occurring at higher levels in select vegetable oils, including palm oil, rice bran oil wheat germ, barley, saw palmetto, anatto, and certain other types of seeds, nuts, grains, and the oils derived from them.[6] High levels of up to 70% occur in palm oil.[7] This vitamin E analogue typically only occurs at very low levels in the human body[8] but different isomers function well as a physical antioxidant.
Chemically, different analogues of vitamin E all show some activity as a chemical antioxidant,[9] but do not all have the same vitamin E equivalence. Alpha-Tocopherol is the form of vitamin E that has the highest biological activity and is preferentially absorbed and accumulated in humans.[2] Its acetate esters are readily hydrolyzed by enzymes (also found in epithelial membranes) and hence are highly bioavailable, however they do not function as physical antioxidants in vitro assays nor in cosmetics / food systems.
Like tocopherols, individual tocotrienol isomers demonstrate different bioavailability[10] and efficacy depending on the type of antioxidant performance being measured.All tocotrienol and tocopherol isomers have some physical antioxidant activity due to an ability to donate a hydrogen atom (a proton plus electron) from the hydroxyl group on the chromanol ring, to free radical and reactive oxygen species. This process inactivates ("quenches") the free radical by donating a single unpaired electron to the radical.
Thus, one function of the lipid soluble vitamin E analogues in the body is that they protect membrane structures in cells and organelles, from free radical damage.
Although analogues have different distributions and metabolic fates the international unit for vitamin E activity was based on pure alpha-tocopherol's capacity for fertility enhancement (by prevention of spontaneous abortions in pregnant rats) This can be alleviated by other analogues including tocotrienols.
Historically studies of tocotrienols account for less than 1% of all research into vitamin E.[11] much of which has focused on alpha-tocopherol. Between 2009 and 2010 ore recently, tocotrienols have been the subject of increased scientific attention, with research on tocotrienols accounting for nearly 30% of all peer-reviewed articles published on vitamin E . The first-ever scientific compilation of tocotrienol research, Tocotrienols: Vitamin E Beyond Tocopherols, was published in 2008 by CRC and AOCS Press, while a second edition was published in 2013.[6]
Etymology
Tocotrienols are named by analogy to tocopherols (from Greek words meaning to bear a pregnancy (see tocopherol); but with this word changed to include the chemical difference that tocotrienols are trienes, meaning that they share identical structure with the tocopherols except for the addition of the three double bonds to their side chains.
History of tocotrienols
The discovery of tocotrienols was first reported by Pennock and Whittle in 1964, describing the isolation of tocotrienols from rubber.[12] The biological significance of tocotrienols was clearly delineated in the early 1980s, when its ability to lower cholesterol was first reported by Qureshi and Elson in the Journal of Medicinal Chemistry.[13] During the 1990s, the anti-cancer properties of tocopherols and tocotrienols began to be delineated.[14]
The current commercial sources of tocotrienol are rice, palm, and annatto.[15] The ratios of tocopherol-to-tocotrienol extracted from rice, palm, and annatto sources are 50:50, 25:75, and 0.1:99.9, respectively. α-tocopherol makes up roughly 25–50% of palm and rice vitamin E mixtures, respectively, and has been shown to interfere with tocotrienol benefits. Annatto, on the other hand, naturally contains only δ- and γ-tocotrienols and is essentially tocopherol-free, hence not confined by α-tocopherol interference issues.
Other natural tocotrienol sources include rice bran oil, coconut oil, cocoa butter, barley, and wheat germ.[16] Sunflower, peanut, walnut, sesame, and olive oils, however contain only tocopherols.[17] Some vitamin E supplements supply 50–200 mg/day of mixed tocotrienols. In a number of clinical trials, doses of tocotrienols as low as 42 mg/day have shown to reduce blood cholesterol levels by 5–35%.[18] Tocotrienols are safe and human studies show no adverse effects with consumption of 240 mg/day for 48 months.[19]
Tocotrienol rich fractions from rice, palm, or annatto, used in nutritional supplements, functional foods, and anti-aging cosmetics, are available in the market at 20%, 35%, 50%, and 70% total vitamin E content. Molecular distillation occurs at lower temperatures and reduces the problem of thermal decomposition. High vacuum also eliminates oxidation that might occur in the presence of air.[20] For the utility and quality it is desired for the absolute tocotrienol concentration to be highest, tocopherol to be the lowest, and the processing solvent-free. Annatto tocotrienol has the highest tocotrienol concentrations, and is tocopherol-free.
Vitamin E, be it tocopherols or tocotrienols, is extremely sensitive to heat.
Comparison of tocotrienol and tocopherol
Since the 1980s, there have been more studies proving tocotrienols are more potent in their anti-oxidation[21] and anti-cancer effect[22][23] than the common forms of tocopherol due to their chemical structure. The unsaturated side-chain in tocotrienols causes them to penetrate tissues with saturated fatty layers more efficiently,[24] making them ideal for anti-aging oral supplements[25] and the skincare range.[26] Tocotrienols are better than tocopherols at combating oxidative stress of skin that had been exposed to UV rays of the sunlight.[27]
Vitamin E has long been known for its antioxidative properties against lipid peroxidation in biological membranes and alpha-tocopherol is considered to be the most active form. In vivo, tocotrienols are more powerful antioxidants, and lipid ORAC values are highest for δ-tocotrienol.[28] However that study also says: "Regarding α-tocopherol equivalent antioxidant capacity no significant differences in the antioxidant activity of all vitamin E isoforms were found." Since 2000, scientists have suggested tocotrienols are better antioxidants than tocopherols[29][30] at preventing cardiovascular diseases[31] and cancer.[32] From the pharmacological standpoint, current formulation of vitamin E supplements, composed mainly of alpha- tocopherol, seems questionable.[33]
Metabolism and bioavailability
The metabolism and thus the bioavailability of tocotrienols are not well understood and simply increasing the intake of tocotrienols might not increase tocotrienol levels in the body.[34]
Tocotrienols are not metabolised by the same mechanisms as tocopherols and tocopherols also interferes with the bioavailability, uptake and functions of tocotrienols.[34]
α-tocopherol interference
Various studies have shown that alpha-tocopherol interferes with tocotrienol benefits.[34] This was first published by Qureshi et al. in 1996, where researchers administered varying amounts of α-tocopherol and/or tocotrienol to six groups of chickens. The group that received only a minimal amount of α-tocopherol showed the greatest reduction in lipid parameters, while the group with the highest amount of alpha-tocopherol had an increase in cholesterol production.[35] A separate study confirmed that high levels of α-tocopherol increase cholesterol production.[36] α-tocopherol interference with tocotrienol absorption was described previously by Ikeda et al., who showed that α-tococopherol interfered with absorption of α-tocotrienol, but not γ-tocotrienol.[37] More recently, Japanese researchers found that tocopherols, and α-tocopherol in particular, interfered with δ-tocotrienol’s ability to induce apoptosis in cancer cells, while blocking the absorption of δ-tocotrienol.[38] Finally, α-tocopherol was shown to interfere with tocotrienols by increasing their catabolism.[39]
Natural sources
In nature, tocotrienols are present in many plants and fruits. Among the edible plants and fruits, the palm fruit (Elaeis guineensis) is particularly high in tocotrienols, primarily gamma-tocotrienol, alpha-tocotrienol and delta-tocotrienol. Other cultivated plants high in tocotrienols includes rice, wheat, barley, rye and oat. Adequate research and basic information on tocotrienol content in plants are generally lacking, but on the basis of our current understanding, tocotrienols tends to be present in much lower amounts than tocopherols in common edible plants.[40] In anatto however, tocotrienols are relatively abundant (only delta- and gamma-tocotrienol) and it contains no tocopherols.[41]
From a human consumption point of view, it is important to understand that even though many common plants are readily available and relatively high in tocotrienols, it might be impossible to raise the tocotrienol levels in the body and obtain a directly measurable health benefit from ordinary consumption alone. To achieve these results it might probably be necessary to apply advanced extraction and concentration techniques. On the other hand, ordinary food processing techniques might actually decrease or completely eliminate the original natural tocotrienol content.
Synthetic tocotrienols
Curiously, synthetic tocotrienol is not commonly available despite the ability to generate the compounds through chemical reactions. Depending on the route of synthesis, the product may result in a racemic mix of dl-tocotrienol which consists of a mix of d and l (left and right) forms that are lateral inversions of one another.[42] However, pure isomers of either the d or l tocotrienol forms should also be possible using the right chemistry. In theory these would then likely confer many of the clinical benefits claimed for natural tocotrienol while being extremely pure in comparison and relatively cheap to produce.
Health effects of tocotrienols
Many research claims of tocotrienols' health benefits for human beings have been made. The toxicity levels for humans are presently unknown. The no-observed-adverse-effect level (NOAEL) for rats is estimated at 120–130 mg/kg body weight/day.[43] As of 2004, the Food and Nutrition Board of the Institute of Medicine of the United States National Academy of Sciences did not define either the health benefits or the health risks, i.e. the Estimated Average Requirement, the Recommended Dietary Allowance, the Adequate Intake and the Tolerable Upper Intake Level (UL) were defined for alpha-tocopherol (except the ULs for infants) but not for tocotrienols.[44]
Tocotrienol is more effective antioxidant than tocopherol because its unsaturated side chain facilitates better penetration into saturated fatty layers of the brain[45] and liver.[46] Tocotrienols can lower tumor formation,[47] DNA damage and cell damage.[48]
Recently, a group from university of Louisiana, showed significant results in that PEGylation of γ-Tocotrienol enhanced its oral bioavialbility significantly when compared with regular marketed formula[49]
Tocotrienols and stroke-induced Injuries
In the peer-reviewed Stroke journal (Oct 2005), oral supplementation of a natural full spectrum palm tocotrienol complex to spontaneously hypertensive rats led to increased tocotrienol levels in the brain. The rats, supplemented with tocotrienols, showed more protection against stroke-induced injury compared to controls (non-supplemented group). This study demonstrated that oral supplementation of the palm tocotrienol complex acts on key molecular checkpoints (c-Src and 12-Lipoxygenase) to protect against glutamate- and stroke-induced neurodegeneration and ultimately protect against stroke in vivo.[50] The protective effects of tocotrienols are independent of their antioxidant activity because tocopherols were effective only at higher concentrates.[51]
In 2005, a study jointly undertaken at Wayne State University and Ohio State University Medical Center showed that tocotrienol can be efficiently delivered to organs and could therefore offer the health benefits suggested by in vitro and in vivo studies.[52] "Our results demonstrate that tocotrienols is efficiently delivered to the bloodstream despite the fact that the transfer protein has a lower affinity for tocotrienols than it has for tocopherols," said Chandan Sen of Ohio State University and senior author of the study.
The researchers recruited women with normal cholesterol levels (average age of 23.5 years old) and gave them a fat-rich strawberry smoothie containing 400 mg of vitamin E containing 77 mg alpha-tocotrienol, 96 mg delta-tocotrienol, and 3 mg gamma-tocotrienol, plus tocopherols. Since vitamin E is a fat-soluble vitamin, the researchers chose to deliver the micronutrient in a fat-loaded meal in order to improve absorption. Blood measurements in the post-prandial period showed that maximal alpha-tocotrienol levels averaged almost 3 micromoles in blood plasma, 1.7 micromoles in low density lipoproteins, and 0.5 micromoles in high density lipoproteins. "This work presented first evidence demonstrating the post-absorptive fate of tocotrienol isomers and their association with lipoprotein subfractions in humans," wrote lead author Pramod Khosla of Wayne State University.
These concentrations, say the researchers, are sufficient to support the proposed neuro-protective functions of tocotrienol. "We have determined that when administered orally, tocotrienol can reach concentrations needed to serve these… protective functions," said Sen. "It is a regular dietary ingredient in Asia, so it can safely be a part of a daily diet within prepared foods or as a supplement in the United States." Can it be therapeutically used to prevent stroke? "Results from animal studies are encouraging, but it is still too soon to tell for humans," he added.
Tocotrienols and white matter lesions
White Matter Lesions (WMLs) are regarded as manifestations of cerebral small vessel disease, reflecting varying degrees of neurodegeneration and tissue damage with potential as a surrogate end point in clinical trials. A total of 121 volunteers aged ≥35 years with cardiovascular risk factors and MRI-confirmed WMLs were randomized to receive 200 mg mixed tocotrienols or placebo twice a day for 2 years. The mean WML volume of the placebo group increased after 2 years, whereas that of the tocotrienol-supplemented group remained essentially unchanged.[53]
Tocotrienols and pancreatic cancer
Pancreatic cancer represents the fourth-leading cause of cancer death in the United States, with a dismal 5-year survival rate of less than 5%. Early detection and screening for pancreatic cancer in the current state should be limited to high-risk patients, although hereditary/familial factors account for only 10% of patients with pancreatic cancer.[54][55]
In a 2009 in-vitro study, scientists at Department of Nutrition and Food Sciences, Texas Woman's University evaluated the impact of d-delta-tocotrienol, on human MIA PaCa-2 and PANC-1 pancreatic carcinoma cells and BxPC-3 pancreatic ductal adenocarcinoma cells. They concluded suppression of mevalonate pathway activities, be it by modulators of HMG CoA reductase (statins, tocotrienols, and farnesol), farnesyl transferase (farnesyl transferase inhibitors), and/or mevalonate pyrophosphate decarboxylase (phenylacetate) activity, have a potential in pancreatic cancer chemotherapy.[56]
Moreover, a Phase I dose-escalating clinical study evaluating the effect of pure δ-tocotrienol towards individuals with pancreatic cancer is running from 2009 to 2013 at the Moffitt Cancer Centre, and is the first tocotrienol study that is being clinically evaluated in humans towards cancer.[57]
Tocotrienols and hepatic cancer
In a 1993 study where rats were induced with a potent liver cancer agent, scientists found less liver cell damage in the group fed with palm tocotrienols.[23][58]
Tocotrienols and breast cancer
In the 1990s, studies showed tocotrienols are the components of vitamin E responsible for growth inhibition in human breast cancer cells in vitro,[59] through estrogen-independent mechanisms.[60] Tocotrienols work synergistically with tamoxifen, a commonly used breast cancer medicine, in killing cancer cells.[61]
Tocotrienols can also affect cell homeostasis, possibly independently of their antioxidant activity.[62] Anti-cancer effects of α- and γ-tocotrienol have been reported, although δ-tocotrienol was verified to be the most effective tocotrienol in inducing apoptosis[63] (cell death) in estrogen-responsive and estrogen-nonresponsive human breast cancer cells. Based on these results on cells in culture, investigators have hypothesised that a mixture of α- and γ-tocotrienols might reduce breast cancer risk.[64]
Further studies on tocotrienol and breast cancer indicated that gamma-tocotrienol targets cancer cells by inhibiting Id1, a key cancer-promoting protein. Gamma-tocotrienol was shown to trigger cell apoptosis as well as anti-proliferation of cancer cells. This mechanism for δ- and γ-tocotrienol was also observed in separate prostate cancer and melanoma cell line studies.[65]
In 2009, a study by scientists at the College of Pharmacy, University of Louisiana at Monroe, showed that lower level statin treatment in combination with γ-tocotrienol inhibits growth of highly malignant +SA mammary epithelial cells in culture (suggesting the possibility of avoiding myotoxicity associated with high dose statin monotherapy).[66]
Tocotrienols and prostate cancer
Investigation of the antiproliferative effect of tocotrienols in PC3 and LNCaP prostate cancer cells suggests that the ability of prostate cancer cells to transform vitamin E to carboxyethyl-hydroxychroman (CEHC) is mostly a detoxification mechanism, useful to maintain the malignant properties of these cells.[67] Various research studies suggest that both δ- and γ-tocotrienol potently suppress prostate cancer cell proliferation.[68] In one study, the antiproliferative effects of γ-tocotrienol were found to act through multiple-signalling pathways (NF-B, EGF-R and Id family proteins). In addition, the same study demonstrated the anti-invasion and chemosensitisation effect of γ-tocotrienol against PCa cells.[69] In another study, δ-tocotrienol was at least equally or more potent than γ-tocotrienol in prostate cancer cells, and showed that alpha-tocopherol enhanced cancer cell growth.[70]
Tocotrienols and skin cancer
In a 2009 study at the Li Ka Shing Faculty of Medicine, The University of Hong Kong, scientists found reduction in skin cancer cells when treated with gamma-tocotrienol with chemotherapy drugs. For the first time, researchers recorded the anti-invasion and chemonsensitization effect of gamma-tocotrienol against human malignant melanoma cells.[71] In cell line and animal studies, δ- and γ- tocotrienols have been shown to suppress the growth of melanoma.[72][73]
Tocotrienols and cholesterol reduction
The human body makes cholesterol from the liver. Tocotrienols can decrease the liver's capacity to manufacture cholesterol. They do so by inhibiting HMG-CoA reductase, the enzyme responsible for cholesterol synthesis.[74]
This was later shown to be due to both the δ- and γ-tocotrienols. Only δ-tocotrienol was shown to block production fully.[75] A 1996 study in chickens indicated that the presence the more abundant alpha-tocopherol may interfere with tocotrienol's ability to lower cholesterol.[35] Since tocotrienols are also converted to tocopherols in vivo, high doses of Tocotrienol may increase total cholesterol. Studies have shown the optimal benefit is obtained with a dose of 100 mg/day of tocotrienol-rich fraction (TRF25).[76]
In 1993, American scientists conducted a double-blind placebo controlled study of 50 volunteers at the Kenneth Jordan Heart Foundation and Elmhurst Medical Center. Their results suggested that tocotrienols (from palm and rice) could ease clogged arteries. Seven high cholesterol patients with narrowing arteries experienced reversal of arterial blockage of the carotid artery after consuming tocotrienols, while in two the condition worsened.[19] This was compared to the control group, where none improved and ten worsened.
An investigation on called FeAOX-6, a synthetic antioxidant which combines structural features of both tocopherols and carotenoids into a single molecule, on macrophage functions involved in foam cell formation showed that both FeAOX-6 and alpha-tocotrienol induce a strong dose-dependent reduction of cholesterol and reduce cholesterol accumulation in human macrophages. The extent of the reduction found with alpha-tocotrienol was greater than that induced by FeAOX-6 and did not correlate with their respective antioxidant capacities.[77]
Tocotrienols and diabetes
According to the World Health Organization (WHO), 170 million people were affected by diabetes in the year 2002, and this number is likely to increase to 366 million by the year 2030.[78] Diabetes mellitus (DM) has been recognized as the sole independent risk factor for the development of any cardiovascular disease.[79] Cardiovascular complications such as stroke and heart attack are increasingly causing death in diabetic patients. Alarmingly, literature statistics indicate that atherosclerosis accounts for about 8 to 10% of all diabetic deaths.[80]
Recent studies are showing that vitamin E intake significantly reduced risk of type 2 diabetes. The relative risk (RR) of type 2 diabetes between the extreme quartiles of the intake was 0.69 (95% CI 0.51-0.94, P for trend=0.003). Intakes of alpha-tocopherol, gamma-tocopherol, delta-tocopherol, and beta-tocotrienol were inversely related to a risk of type 2 diabetes. While correlation does not imply causation, these data suggest the possibility that the development of type 2 diabetes might be modified by the intake of antioxidants in the diet.[81]
In 2009, animal trials carried out in India and Malaysia revealed palm tocotrienols improved blood glucose, dyslipidemia and oxidative stress in diabetic rats. It is able to prevent the progression of vascular wall changes occurring in DM.[82][83] Moreover, tocotrienols alone and in mixture with alpha-tocopherol had the capability to enhance lymphocyte proliferation among streptozotocin-induced diabetic rats.[84]
Tocotrienols as radiation countermeasures
Following exposure to gamma radiation, hematopoietic stem cells (HSCs) in the bone marrow, which are important for producing blood cells, rapidly undergo apoptosis (cell death). There are no known treatments for this acute effect of radiation.[85] Two studies conducted by researchers at the U.S. Armed Forces Radiobiology Research Institute (AFRRI) found that treatment with γ-tocotrienol or δ-tocotrienol significantly enhanced survival of hematopoietic stem cells, which are essential for renewing the body's supply of blood cells.[85][86]
In one study, after exposure to total-body irradiation, the number of hematopoietic progenitor cells (HPCs) in mice treated with γ-tocotrienol recovered by 90% after 7 days while HPC counts of the mice in the control group, which were treated identically except that they did not receive any form of tocotrienol, failed to recover by more than 30%, even after 13 days.[86] In another study, two groups of mice were exposed to a large dose of gamma radiation. One day before the radiation exposure, one group of mice was injected with a massive dose (400 mg / kg) of δ-tocotrienol while the other group—the control group—was injected with the liquid delivery vehicle without any tocotrienol content. Thirty days after exposure to the radiation, 100% of the mice that had received the δ-tocotrienol injection were still alive, but only 18% of the mice in the control group, which did not receive the tocotrienol inject, were still living. In addition, testing performed not on the mice found that the mice treated with δ-tocotrienol had both higher rates of survival and regeneration of important hematopoietic progenitor cells (HPCs).[85] In the same investigation, the researchers also assessed the survival of human hematopoietic progenitor CD34+ cells when treated in vitro with δ-tocotrienol either 24 hours before or 6 hours after exposure to a dose of gamma irradiation. They found that treatment with δ-tocotrienol 24 hours before irradiation significantly increased the number of human CD34+ cells surviving 3 days later. However, treatment with δ-tocotrienol that was administered 6 hours after irradiation made no significant difference in the number of human CD34+ cells surviving.[85] The researchers found that the radio-protetive effect of δ-tocotrienol is due to δ-tocotrienol-induced phosphorylation of extracellular signal-regulated kinases (ERKs) on human CD34+ and mice bone marrow cells. ERK activation is one component of the MAPK/ERK pathway (mitogen-activated protein kinases/extracellular signal-regulated kinases pathway), which activates DNA repair and cell growth.[85]
A 2014 AFRRI study conducted using irradiated mice found that δ-tocotrienol administration induced higher levels of cytokines than other tocols being studied as radiation countermeasures. Most significantly, the radioprotective effects of δ-tocotrienol appear to be mediated by granulocyte colony-stimulating factor (G-CSF). When irradiated mice treated with δ-tocotrienol were subsequently injected with a G-CSF neutralizing antibody, the radioprotective effects conferred by δ-tocotrienol were completely abrogated.[87]
A 2009 study conducted at AFRRI sought to evaluate the radio-protective potential of γ-tocotrienol including how best to optimize the treatment regimen in terms of both time and dose. By conducting a series of experiments on irradiated mice, the researchers found that a dose of 200 mg/kg given by subcutaneous injection (SC) 24 hours before radiation exposure had a dose reduction factor of 1.29.[88] Based on these successful results of studies in mice, γ-tocotrienol is being studied for its safety and efficacy as a radioprotective measure in nonhuman primates.[89]
No-observed-adverse-effect level
A 13-week study by H. Nakamura and colleagues at the National Institute of Health Sciences (Japan) of tocotrienols' toxicity in rats found significant changes in several blood components, increases in liver weights and (in females) reductions in ovary and uterus weights, depending on the dosages. The authors estimated the no-observed-adverse-effect level (NOAEL) to be 120 mg per kg of body weight per day for males and 130 mg per kg of body weight per day for females. Since effects on the blood components were observed in all cases with non-placebos, a no-observed-effect level (NOEL) could not be determined.[43]
See also
References
- ↑ Whittle KJ, Dunphy PJ, Pennock JF (July 1966). "The isolation and properties of δ-tocotrienol from Hevea latex". The Biochemical Journal. 100 (1): 138–45. PMC 1265104
 . PMID 5965249.
. PMID 5965249. - ↑ Brigelius-Flohé R, Traber MG (July 1999). "Vitamin E: function and metabolism". The FASEB Journal. 13 (10): 1145–55. PMID 10385606.
- ↑ Muller (2010). "Mol Nutr Food Res.". In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma.
- ↑ Kamal-Eldin A, Appelqvist LA (July 1996). "The chemistry and antioxidant properties of tocopherols and tocotrienols". Lipids. 31 (7): 671–701. doi:10.1007/BF02522884. PMID 8827691.
- ↑ Clarke MW, Burnett JR, Croft KD (2008). "Vitamin E in human health and disease". Critical Reviews in Clinical Laboratory Sciences. 45 (5): 417–50. doi:10.1080/10408360802118625. PMID 18712629.
- 1 2 Tan, B; Watson, RR; Preedy, VR (2013), Tocotrienols: Vitamin E Beyond Tocopherols (2nd ed.), Boca Raton: CRC Press, ISBN 9781439884416
- ↑ Sen, Chandran (June 2010). "Palm Oil–Derived Natural Vitamin E α-Tocotrienol in Brain Health and Disease". J Am Coll Nutr. Retrieved 2016-09-28.
- ↑ Packer, Lester; Fuchs, Jürgen (1993). Vitamin E in health and disease. CRC Press. p. 3. ISBN 978-0-8247-8692-2.
- ↑ Cerecetto H, López GV (March 2007). "Antioxidants derived from vitamin E: an overview". Mini Reviews in Medicinal Chemistry. 7 (3): 315–38. doi:10.2174/138955707780059871. PMID 17346221.
- ↑ "https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-11-5". Nutrition & Metabolism. 11:5. External link in
|title=(help) - ↑ Sen CK, Khanna S, Roy S (2007). "Tocotrienols in health and disease: the other half of the natural vitamin E family". Molecular Aspects of Medicine. 28 (5–6): 692–728. doi:10.1016/j.mam.2007.03.001. PMC 2435257
 . PMID 17507086.
. PMID 17507086. - ↑ Dunphy, P. J.; Whittle, K. J.; Pennock, J. F.; Morton, R. A. (1965). "Identification and Estimation of Tocotrienols in Hevea Latex". Nature. 207 (4996): 521–522. doi:10.1038/207521a0.
- ↑ Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ (October 1992). "Hypocholesterolemic activity of synthetic and natural tocotrienols". J. Med. Chem. 35 (20): 3595–606. doi:10.1021/jm00098a002. PMID 1433170.
- ↑ Watson & Preedy 2008, p. 6
- ↑ Tan, B. and M.H. Saleh, Integrated process for recovery of carotenoids and tocotrienols from oil in USPTO 5,157,132. 1992
- ↑ Packer L, Weber SU, Rimbach G (February 2001). "Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling". The Journal of Nutrition. 131 (2): 369S–73S. PMID 11160563.
- ↑ Heinonen M, Piironen V (1991). "The tocopherol, tocotrienol, and vitamin E content of the average Finnish diet". International Journal for Vitamin and Nutrition Research. 61 (1): 27–32. PMID 1856041.
- ↑ Tan DT, Khor HT, Low WH, Ali A, Gapor A (April 1991). "Effect of a palm-oil-vitamin E concentrate on the serum and lipoprotein lipids in humans". The American Journal of Clinical Nutrition. 53 (4 Suppl): 1027S–1030S. PMID 2012011.
- 1 2 Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML (December 1995). "Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis". Lipids. 30 (12): 1179–83. doi:10.1007/BF02536621. PMID 8614310.
- ↑ Liu, Donghong; Shi, John; Posada, Luidy Rodriguez; Kakuda, Yukio; Xue, Sophia Jun (2008). "Separating Tocotrienols from Palm Oil by Molecular Distillation". Food Reviews International. 24 (4): 376–391. doi:10.1080/87559120802303840.
- ↑ Serbinova E, Kagan V, Han D, Packer L (1991). "Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol". Free Radical Biology & Medicine. 10 (5): 263–75. doi:10.1016/0891-5849(91)90033-Y. PMID 1649783.
- ↑ Constantinou C, Papas A, Constantinou AI (August 2008). "Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs". International Journal of Cancer. 123 (4): 739–52. doi:10.1002/ijc.23689. PMID 18512238.
- 1 2 Wada S (2009). "Chemoprevention of tocotrienols: the mechanism of antiproliferative effects". Forum of Nutrition. Forum of Nutrition. 61: 204–16. doi:10.1159/000212752. ISBN 978-3-8055-9097-6. PMID 19367124.
- ↑ Suzuki YJ, Tsuchiya M, Wassall SR, et al. (October 1993). "Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency". Biochemistry. 32 (40): 10692–9. doi:10.1021/bi00091a020. PMID 8399214.
- ↑ Rona C, Vailati F, Berardesca E (January 2004). "The cosmetic treatment of wrinkles". Journal of Cosmetic Dermatology. 3 (1): 26–34. doi:10.1111/j.1473-2130.2004.00054.x. PMID 17163944.
- ↑ Traber MG, Rallis M, Podda M, Weber C, Maibach HI, Packer L (January 1998). "Penetration and distribution of alpha-tocopherol, alpha- or gamma-tocotrienols applied individually onto murine skin". Lipids. 33 (1): 87–91. doi:10.1007/s11745-998-0183-0. PMID 9470177.
- ↑ Weber C, Podda M, Rallis M, Thiele JJ, Traber MG, Packer L (1997). "Efficacy of topically applied tocopherols and tocotrienols in protection of murine skin from oxidative damage induced by UV-irradiation". Free Radical Biology & Medicine. 22 (5): 761–9. doi:10.1016/S0891-5849(96)00346-2. PMID 9119243.
- ↑ Müller L, Theile K, Böhm V (May 2010). "In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma". Mol Nutr Food Res. 54 (5): 731–42. doi:10.1002/mnfr.200900399. PMID 20333724.
- ↑ Yoshida Y, Niki E, Noguchi N (March 2003). "Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects". Chemistry and Physics of Lipids. 123 (1): 63–75. doi:10.1016/S0009-3084(02)00164-0. PMID 12637165.
- ↑ Schaffer S, Müller WE, Eckert GP (February 2005). "Tocotrienols: constitutional effects in aging and disease". The Journal of Nutrition. 135 (2): 151–4. PMID 15671205.
- ↑ Pruthi S, Allison TG, Hensrud DD (November 2001). "Vitamin E supplementation in the prevention of coronary heart disease". Mayo Clinic Proceedings. 76 (11): 1131–6. doi:10.4065/76.11.1131. PMID 11702901.
- ↑ Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M, Miyazawa T (July 2003). "Anti-angiogenic activity of tocotrienol". Bioscience, Biotechnology, and Biochemistry. 67 (7): 1623–7. doi:10.1271/bbb.67.1623. PMID 12913317.
- ↑ Theriault A, Chao JT, Wang Q, Gapor A, Adeli K (July 1999). "Tocotrienol: a review of its therapeutic potential". Clinical Biochemistry. 32 (5): 309–19. doi:10.1016/S0009-9120(99)00027-2. PMID 10480444.
- 1 2 3 Fu, J. Y.; Che, H. L.; Tan, D. M.; Teng, K. T. (2014). "Bioavailability of tocotrienols: Evidence in human studies". Nutrition & Metabolism. 11 (1): 5. doi:10.1186/1743-7075-11-5. PMC 3895660
 . PMID 24410975.
. PMID 24410975. - 1 2 Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE (February 1996). "Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens". The Journal of Nutrition. 126 (2): 389–94. PMID 8632210.
- ↑ Stocker A (December 2004). "Molecular mechanisms of vitamin E transport". Ann. N. Y. Acad. Sci. 1031: 44–59. doi:10.1196/annals.1331.005. PMID 15753133.
- ↑ Ikeda S, Tohyama T, Yoshimura H, Hamamura K, Abe K, Yamashita K (February 2003). "Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats". J. Nutr. 133 (2): 428–34. PMID 12566479.
- ↑ Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Asai A, Miyazawa T (June 2010). "alpha-Tocopherol attenuates the cytotoxic effect of delta-tocotrienol in human colorectal adenocarcinoma cells". Biochem. Biophys. Res. Commun. 397 (2): 214–9. doi:10.1016/j.bbrc.2010.05.087. PMID 20493172.
- ↑ Sontag TJ, Parker RS (May 2007). "Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase". J. Lipid Res. 48 (5): 1090–8. doi:10.1194/jlr.M600514-JLR200. PMID 17284776.
- ↑ Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet p.199
- ↑ Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry
- ↑ Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ (October 1992). "Hypocholesterolemic activity of synthetic and natural tocotrienols". Journal of Medicinal Chemistry. 35 (20): 3595–606. doi:10.1021/jm00098a002. PMID 1433170.
- 1 2 Nakamura H, Furukawa F, Nishikawa A, et al. (August 2001). "Oral toxicity of a tocotrienol preparation in rats". Food Chem. Toxicol. 39 (8): 799–805. doi:10.1016/S0278-6915(01)00025-4. PMID 11434987.
- ↑ Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Food and Nutrition Board, Institute of Medicine, National Academies, 2004, retrieved 2009-06-09
- ↑ Kamat JP, Devasagayam TP (August 1995). "Tocotrienols from palm oil as potent inhibitors of lipid peroxidation and protein oxidation in rat brain mitochondria". Neuroscience Letters. 195 (3): 179–82. doi:10.1016/0304-3940(95)11812-B. PMID 8584204.
- ↑ Kamat JP, Sarma HD, Devasagayam TP, Nesaretnam K, Basiron Y (May 1997). "Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes". Molecular and Cellular Biochemistry. 170 (1–2): 131–7. doi:10.1023/A:1006853419214. PMID 9144327.
- ↑ Weng-Yew W, Selvaduray KR, Ming CH, Nesaretnam K (2009). "Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis". Nutrition and Cancer. 61 (3): 367–73. doi:10.1080/01635580802582736. PMID 19373610.
- ↑ Chin SF, Hamid NA, Latiff AA, et al. (January 2008). "Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation". Nutrition. 24 (1): 1–10. doi:10.1016/j.nut.2007.08.006. PMID 17884341.
- ↑ Abu-Fayyad, Ahmed; Behery, Fathy; Sallam, Asmaa A.; Alqahtani, Saeed; Ebrahim, Hassan; El Sayed, Khalid A.; Kaddoumi, Amal; Sylvester, Paul W.; Carroll, Jennifer L. (2015-10-01). "PEGylated γ-tocotrienol isomer of vitamin E: Synthesis, characterization, in vitro cytotoxicity, and oral bioavailability". European Journal of Pharmaceutics and Biopharmaceutics. 96: 185–195. doi:10.1016/j.ejpb.2015.07.022.
- ↑ Khanna S, Roy S, Slivka A, et al. (October 2005). "Neuroprotective Properties of The Natural Vitamin E α-Tocotrienol". Stroke. 36 (10): 2258–64. doi:10.1161/01.STR.0000181082.70763.22. PMC 1829173
 . PMID 16166580.
. PMID 16166580. - ↑ Sen CK, Khanna S, Roy S, Packer L (April 2000). "Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells". The Journal of Biological Chemistry. 275 (17): 13049–55. doi:10.1074/jbc.275.17.13049. PMID 10777609.
- ↑ Khosla P, Patel V, Whinter JM, et al. (2006). "Postprandial levels of the natural vitamin E tocotrienol in human circulation". Antioxidants & Redox Signaling. 8 (5–6): 1059–68. doi:10.1089/ars.2006.8.1059. PMID 16771695.
- ↑ Yogheswaran Gopalan, et al, (May 2014). "Clinical Investigation of the Protective Effects of Palm Vitamin E Tocotrienols on Brain White Matter.". Stroke. 45: 1422–1428. doi:10.1161/STROKEAHA.113.004449.
- ↑ Klapman J, Malafa MP (October 2008). "Early detection of pancreatic cancer: why, who, and how to screen" (PDF). Cancer Control. 15 (4): 280–7. PMID 18813195.
- ↑ Malafa MP (October 2008). "New insights and gains in pancreatic cancer" (PDF). Cancer Control. 15 (4): 276–7. PMID 18813194.
- ↑ Hussein D, Mo H (May 2009). "d-Dlta-tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells". Pancreas. 38 (4): e124–36. doi:10.1097/MPA.0b013e3181a20f9c. PMID 19346993.
- ↑ Clinical trial number NCT00985777 for "Vitamin E δ-Tocotrienol Administered to Subjects With Resectable Pancreatic Exocrine Neoplasia" at ClinicalTrials.gov
- ↑ Rahmat A, Ngah WZ, Shamaan NA, Gapor A, Abdul Kadir K (1993). "Long-term administration of tocotrienols and tumor-marker enzyme activities during hepatocarcinogenesis in rats". Nutrition. 9 (3): 229–32. PMID 8102564.
- ↑ Nesaretnam K, Guthrie N, Chambers AF, Carroll KK (December 1995). "Effect of tocotrienols on the growth of a human breast cancer cell line in culture". Lipids. 30 (12): 1139–43. doi:10.1007/BF02536615. PMID 8614304.
- ↑ Nesaretnam K, Stephen R, Dils R, Darbre P (May 1998). "Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status". Lipids. 33 (5): 461–9. doi:10.1007/s11745-998-0229-3. PMID 9625593.
- ↑ Guthrie N, Gapor A, Chambers AF, Carroll KK (March 1997). "Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination". The Journal of Nutrition. 127 (3): 544S–548S. PMID 9082043.
- ↑ Yu W, Simmons-Menchaca M, Gapor A, Sanders BG, Kline K (1999). "Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols". Nutrition and Cancer. 33 (1): 26–32. doi:10.1080/01635589909514744. PMID 10227040.
- ↑ Sylvester, Paul W.; Shah, Sumit (2002). "Antioxidants in Dietary Oils: Their Potential Role in Breast Cancer Prevention". Malaysian Journal of Nutrition. 8 (1): 1–11. ISSN 1394-035X.
- ↑ Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Canali R, Virgili F (December 2004). "Tocotrienol-rich fraction from palm oil and gene expression in human breast cancer cells". Ann. N. Y. Acad. Sci. 1031: 143–57. doi:10.1196/annals.1331.014. PMID 15753141.
- ↑ Yap WN, Zaiden N, Tan YL, Ngoh CP, Zhang XW, Wong YC, Ling MT, Yap YL (November 2009). "Id1, inhibitor of differentiation, is a key protein mediating anti-tumor responses of gamma-tocotrienol in breast cancer cells". Cancer Lett. 291 (2): 187–99. doi:10.1016/j.canlet.2009.10.012. PMID 19926394.
- ↑ Wali VB, Bachawal SV, Sylvester PW (June 2009). "Combined treatment of gamma-tocotrienol with statins induce mammary tumor cell cycle arrest in G1". Experimental Biology and Medicine. 234 (6): 639–50. doi:10.3181/0810-RM-300. PMID 19359655.
- ↑ Conte C, Floridi A, Aisa C, Piroddi M, Floridi A, Galli F (December 2004). "Gamma-tocotrienol metabolism and antiproliferative effect in prostate cancer cells". Ann. N. Y. Acad. Sci. 1031: 391–4. doi:10.1196/annals.1331.054. PMID 15753178.
- ↑ Constantinou C, Hyatt JA, Vraka PS, et al. (2009). "Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives". Nutr Cancer. 61 (6): 864–74. doi:10.1080/01635580903285130. PMID 20155628.
- ↑ Yap WN, Chang PN, Han HY, et al. (December 2008). "γ-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways". British Journal of Cancer. 99 (11): 1832–41. doi:10.1038/sj.bjc.6604763. PMC 2600692
 . PMID 19002171.
. PMID 19002171. - ↑ Campbell SE, Rudder B, Phillips RB, et al. (May 2011). "γ-Tocotrienol induces growth arrest through a novel pathway with TGFβ2 in prostate cancer". Free Radic. Biol. Med. 50 (10): 1344–54. doi:10.1016/j.freeradbiomed.2011.02.007. PMID 21335085.
- ↑ Chang PN, Yap WN, Lee DT, Ling MT, Wong YC, Yap YL (2009). "Evidence of gamma-tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells". Nutrition and Cancer. 61 (3): 357–66. doi:10.1080/01635580802567166. PMID 19373609.
- ↑ He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE (May 1997). "Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo". J. Nutr. 127 (5): 668–74. PMID 9164984.
- ↑ McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H (April 2007). "Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo". Exp. Biol. Med. (Maywood). 232 (4): 523–31. PMID 17392488.
- ↑ Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ (May 1993). "Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase". The Journal of Biological Chemistry. 268 (15): 11230–8. PMID 8388388.
- ↑ Song BL, DeBose-Boyd RA (September 2006). "Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols". The Journal of Biological Chemistry. 281 (35): 25054–61. doi:10.1074/jbc.M605575200. PMID 16831864.
- ↑ Qureshi AA1, Sami SA, Salser WA, Khan FA. (March 2002). "Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans.". Atherosclerosis. 161: 199–207. doi:10.1016/s0021-9150(01)00619-0. PMID 11882333.
- ↑ Napolitano M, Avanzi L, Manfredini S, Bravo E (June 2007). "Effects of new combinative antioxidant FeAOX-6 and alpha-tocotrienol on macrophage atherogenesis-related functions". Vascul. Pharmacol. 46 (6): 394–405. doi:10.1016/j.vph.2006.01.019. PMID 17331802.
- ↑ Wild S, Roglic G, Green A, Sicree R, King H (May 2004). "Global prevalence of diabetes: estimates for the year 2000 and projections for 2030". Diabetes Care. 27 (5): 1047–53. doi:10.2337/diacare.27.5.1047. PMID 15111519.
- ↑ Klein R (February 1995). "Hyperglycemia and microvascular and macrovascular disease in diabetes". Diabetes Care. 18 (2): 258–68. doi:10.2337/diacare.18.2.258. PMID 7729308.
- ↑ Gu K, Cowie CC, Harris MI (July 1998). "Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993". Diabetes Care. 21 (7): 1138–45. doi:10.2337/diacare.21.7.1138. PMID 9653609.
- ↑ Montonen J, Knekt P, Järvinen R, Reunanen A (February 2004). "Dietary antioxidant intake and risk of type 2 diabetes". Diabetes Care. 27 (2): 362–6. doi:10.2337/diacare.27.2.362. PMID 14747214.
- ↑ Kuhad A, Bishnoi M, Tiwari V, Chopra K (April 2009). "Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits". Pharmacology, Biochemistry, and Behavior. 92 (2): 251–9. doi:10.1016/j.pbb.2008.12.012. PMID 19138703.
- ↑ Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J (June 2009). "The Effects of Palm Oil Tocotrienol-Rich Fraction Supplementation on Biochemical Parameters, Oxidative Stress and the Vascular Wall of Streptozotocin-Induced Diabetic Rats". Clinics. 64 (3): 235–44. doi:10.1590/S1807-59322009000300015. PMC 2666447
 . PMID 19330251.
. PMID 19330251. - ↑ Mojani, Mansooreh Sadat; Vahid Hosseinpour Sarmadi; Asmah Rahmat; Rajesh Ramasamy (2013-06-18). "Effect of Palm Tocotrienols versus Alpha-Tocopherol on Lymphocyte's Proliferation in Streptozotocin-Induced Diabetic Rats" (PDF). Research inImmunology: An International Journal: 1–9. doi:10.5171/2013.189211.
- 1 2 3 4 5 Li XH, Fu D, Latif NH, et al. (December 2010). "δ-tocotrienol protects mouse and human hematopoietic progenitors from γ-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling". Haematologica. 95 (12): 1996–2004. doi:10.3324/haematol.2010.026492. PMC 2995556
 . PMID 20823133.
. PMID 20823133. - 1 2 Kulkarni S, Ghosh SP, Satyamitra M, et al. (June 2010). "Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation". Radiat. Res. 173 (6): 738–47. doi:10.1667/RR1824.1. PMID 20518653.
- ↑ Singh VK, Wise SY, Scott JR, et al. (28 Jan 2014). "Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor.". Life Sci. 98 (2): 113–22. doi:10.1016/j.lfs.2014.01.065. PMID 24486300.
- ↑ Ghosh SP, Kulkarni S, Hieber K, et al. (July 2009). "Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector". Int. J. Radiat. Biol. 85 (7): 598–606. doi:10.1080/09553000902985128. PMID 19557601.
- ↑ Singh VK, Beattie LA, Seed TM (Nov 1, 2013). "Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures". Journal of Radiation Research. 54 (6): 973–988. doi:10.1093/jrr/rrt048. PMID 23658414.
External links
- Vitamin E factsheet — Office of Dietary Supplements, National Institutes of Health
- Tocotrienols at the US National Library of Medicine Medical Subject Headings (MeSH)
- Watson, Ronald R.; Preedy, Victor R., eds. (2008). Tocotrienols: Vitamin E beyond Tocopherols. Boca Raton: CRC Press. ISBN 978-1-4200-8037-7.