Time stretch quantitative phase imaging
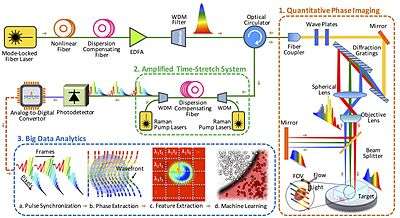
Time-stretch quantitative phase imaging (TS-QPI) is an imaging technique based on time-stretch technology for simultaneous measurement of phase and intensity spatial profiles.[1][2][3][4] Developed at UCLA, it has led to the development of time stretch artificial intelligence microscope.[1]
Time stretched imaging
In time stretched imaging, the object’s spatial information is encoded in the spectrum of laser pulses within a pulse duration of sub-nanoseconds. Each pulse representing one frame of the camera is then stretched in time so that it can be digitized in real-time by an electronic analog-to-digital converter (ADC). The ultra-fast pulse illumination freezes the motion of high-speed cells or particles in flow to achieve blur-free imaging. Detection sensitivity is challenged by the low number of photons collected during the ultra-short shutter time (optical pulse width) and the drop in the peak optical power resulting from the time stretch. These issues are solved in time stretch imaging by implementing a low noise-figure Raman amplifier within the dispersive device that performs time stretching. Moreover, warped stretch transform can be used in time stretch imaging to achieve optical image compression and nonuniform spatial resolution over the field-of-view.
In the coherent version of the time-stretch camera, the imaging is combined with spectral interferometry to measure quantitative phase[5][6] and intensity images in real-time and at high throughput. Integrated with a microfluidic channel, coherent time stretch imaging system measures both quantitative optical phase shift and loss of individual cells as a high-speed imaging flow cytometer, capturing millions of line-images per second in flow rates as high as a few meters per second, reaching up to hundred-thousand cells per second throughput. The time stretch quantitative phase imaging can be combined with machine learning to achieve very accurate label-free classification of the cells.
References
- 1 2 Chen, Claire Lifan; Mahjoubfar, Ata; Tai, Li-Chia; Blaby, Ian K.; Huang, Allen; Niazi, Kayvan Reza; Jalali, Bahram (2016). "Deep Learning in Label-free Cell Classification". Scientific Reports. Full text download available. 6: 21471. Bibcode:2016NatSR...621471C. doi:10.1038/srep21471. PMC 4791545
 . PMID 26975219.published under CC BY 4.0 licensing
. PMID 26975219.published under CC BY 4.0 licensing - ↑ Michaud, Sarah (5 April 2016). "Leveraging Big Data for Cell Imaging". Optics & Photonics News. The Optical Society. Retrieved 8 July 2016.
- ↑ "Photonic Time Stretch Microscopy Combined with Artificial Intelligence Spots Cancer Cells in Blood". Med Gadget. 15 April 2016. Retrieved 8 July 2016.
- ↑ Chen, Claire Lifan; Mahjoubfar, Ata; Jalali, Bahram (23 April 2015). "Optical Data Compression in Time Stretch Imaging". PLoS ONE. Full text download available. 10 (4): e0125106. doi:10.1371/journal.pone.0125106. PMC 4408077
 . PMID 25906244.
. PMID 25906244. - ↑ G. Popescu, "Quantitative phase imaging of cells and tissues," McGraw Hill Professional (2011)
- ↑ Lau, Andy K. S.; Wong, Terence T. W.; Ho, Kenneth K. Y.; Tang, Matthew T. H.; Chan, Antony C. S.; Wei, Xiaoming; Lam, Edmund Y.; Shum, Ho Cheung; Wong, Kenneth K. Y.; Tsia, Kevin K. (2014). "Interferometric time-stretch microscopy for...quantitative cellular and tissue imaging." (PDF). Journal of Biomedical Optics. Free PDF download. 19 (7): 076001. Bibcode:2014JBO....19g6001L. doi:10.1117/1.JBO.19.7.076001.