Thyroglobulin
| TG | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | TG, AITD3, TGN, thyroglobulin | ||||||||||||||||
| External IDs | OMIM: 188450 MGI: 98733 HomoloGene: 2430 GeneCards: TG | ||||||||||||||||
| |||||||||||||||||
| RNA expression pattern | |||||||||||||||||
   | |||||||||||||||||
| More reference expression data | |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 8: 132.87 – 133.13 Mb | Chr 15: 66.67 – 66.85 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
Thyroglobulin (Tg) is a 660 kDa, dimeric protein produced by the follicular cells of the thyroid and used entirely within the thyroid gland. Thyroglobulin protein accounts for approximately half of the protein content of the thyroid gland.[3] Human TG is a homodimer of subunits each containing 2768 amino acids as synthesized (a short signal peptide may be removed from the N-terminus in the mature protein).[4]
The protein is a precursor of the thyroid hormones; these are produced when thyroglobulin's tyrosine residues are combined with iodine and the protein is subsequently cleaved. Each thyroglobulin molecule contains approximately 100-120 tyrosine residues, but only a small number (20) of these are subject to iodination by thyroperoxidase in the follicular colloid. Therefore, each Tg molecule forms only approximately 10 thyroid hormone molecules.[3]
Function
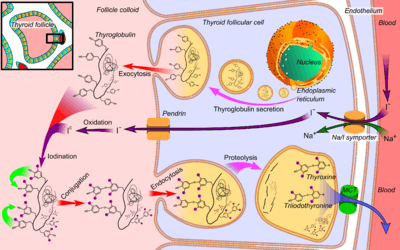
Tg is used by the thyroid gland to produce the thyroid hormones thyroxine (T4) and triiodothyronine (T3). The active form of triiodothyronine, 3, 5, 3' triiodothyronine, is produced both within the thyroid gland and in the periphery by 5'-deiodinase (which has been referred to as tetraiodothyronine 5' deiodinase). It is presumed that Tg and thyroid are also an important storage of iodine for all body needs, in particular, for many iodine-concentrating organs such as breast, stomach, salivary glands, thymus, choroid plexus and cerebrospinal fluid, etc. (see iodine in biology).[5]
Tg is produced by the thyroid epithelial cells, called thyrocytes, which form spherical follicles. Tg is secreted and stored in the follicular lumen.
Via a reaction with the enzyme thyroperoxidase, iodine is covalently bound to tyrosine residues in thyroglobulin molecules, forming monoiodotyrosine (MIT) and diiodotyrosine (DIT).
- Thyroxine is produced by combining two moieties of DIT.
- Triiodothyronine is produced by combining one molecule of MIT and one molecule of DIT.
Small globules of the follicular colloid (Tg) are endocytosed (hormone (TSH)-mediated) and proteases in lysosomes digest iodinated thyroglobulin, releasing T3 and T4 within the thyrocyte cytoplasm. The T3 and T4 are then transported across (TSH-mediated) the basolateral thyrocyte membrane, into the bloodstream, by an unknown mechanism, while the lysosome is recycled back to the follicular lumen.
Clinical significance
Half-life and clinical elevation
Metabolism of thyroglobulin occurs in the liver and via thyroid gland recycling of the protein. Circulating thyroglobulin has a half-life of 65 hours. Following thyroidectomy, it may take many weeks before thyroglobulin levels become undetectable. After thyroglobulin levels become undetectable (following thyroidectomy), levels can be serially monitored.
A subsequent elevation of the thyroglobulin level is an indication of recurrence of papillary or follicular thyroid carcinoma. Hence, thyroglobulin levels in the blood are mainly used as a tumor marker[6] for certain kinds of thyroid cancer (particularly papillary or follicular thyroid cancer). Thyroglobulin is not produced by medullary or anaplastic thyroid carcinoma.
Thyroglobulin antibodies
In the clinical laboratory, thyroglobulin testing can be complicated by the presence of anti-thyroglobulin antibodies (ATAs), alternatively referred to as TgAb. Anti-thyroglobulin antibodies are present in 1 in 10 normal individuals, and a greater percentage of patients with thyroid carcinoma. The presence of these antibodies can result in falsely low (or rarely falsely high) levels of reported thyroglobulin, a problem that can be somewhat circumvented by concomitant testing for the presence of ATAs. The ideal strategy for a clinician's interpretation and management of patient care in the event of confounding detection of ATAs is testing to follow serial quantitative measurements (rather than a single laboratory measurement).
ATAs are often found in patients with Hashimoto's thyroiditis or Graves' disease. Their presence is of limited use in the diagnosis of these diseases, since they may also be present in healthy euthyroid individuals. ATAs are also found in patients with Hashimoto's encephalopathy, a neuroendocrine disorder related to—but not caused by—Hashimoto's thyroiditis.[7]
Interactions
Thyroglobulin has been shown to interact with Binding immunoglobulin protein.[8][9]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 Boron WF (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1044. ISBN 1-4160-2328-3.
- ↑ ((cite web |url="http://www.ncbi.nlm.nih.gov/protein/NP_003226.4"))
- ↑ Venturi S, Donati FM, Venturi A, Venturi M (August 2000). "Environmental iodine deficiency: A challenge to the evolution of terrestrial life?". Thyroid. 10 (8): 727–9. doi:10.1089/10507250050137851. PMID 11014322.
- ↑ "ACS :: Tumor Markers". American Cancer Society. Retrieved 2009-03-28.
- ↑ Ferracci F, Moretto G, Candeago RM, Cimini N, Conte F, Gentile M, Papa N, Carnevale A (February 2003). "Antithyroid antibodies in the CSF: Their role in the pathogenesis of Hashimoto's encephalopathy". Neurology. 60 (4): 712–4. doi:10.1212/01.wnl.0000048660.71390.c6. PMID 12601119.
- ↑ Delom F, Mallet B, Carayon P, Lejeune PJ (June 2001). "Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein". J. Biol. Chem. 276 (24): 21337–42. doi:10.1074/jbc.M101086200. PMID 11294872.
- ↑ Delom F, Lejeune PJ, Vinet L, Carayon P, Mallet B (February 1999). "Involvement of oxidative reactions and extracellular protein chaperones in the rescue of misassembled thyroglobulin in the follicular lumen". Biochem. Biophys. Res. Commun. 255 (2): 438–43. doi:10.1006/bbrc.1999.0229. PMID 10049727.
Further reading
- Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, Haugen BR, Sherman SI, Cooper DS, Braunstein GD, Lee S, Davies TF, Arafah BM, Ladenson PW, Pinchera A (2003). "A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma". J. Clin. Endocrinol. Metab. 88 (4): 1433–41. doi:10.1210/jc.2002-021702. PMID 12679418.
- Henry M, Zanelli E, Piechaczyk M, Pau B, Malthièry Y (1992). "A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies". Eur. J. Immunol. 22 (2): 315–9. doi:10.1002/eji.1830220205. PMID 1371467.
- Targovnik HM, Cochaux P, Corach D, Vassart G (1992). "Identification of a minor Tg mRNA transcript in RNA from normal and goitrous thyroids". Mol. Cell. Endocrinol. 84 (1-2): R23–6. doi:10.1016/0303-7207(92)90087-M. PMID 1639210.
- Dunn AD, Crutchfield HE, Dunn JT (1991). "Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L". J. Biol. Chem. 266 (30): 20198–204. PMID 1939080.
- Lamas L, Anderson PC, Fox JW, Dunn JT (1989). "Consensus sequences for early iodination and hormonogenesis in human thyroglobulin". J. Biol. Chem. 264 (23): 13541–5. PMID 2760035.
- Marriq C, Lejeune PJ, Venot N, Vinet L (1989). "Hormone synthesis in human thyroglobulin: possible cleavage of the polypeptide chain at the tyrosine donor site". FEBS Lett. 242 (2): 414–8. doi:10.1016/0014-5793(89)80513-7. PMID 2914619.
- Christophe D, Cabrer B, Bacolla A, Targovnik H, Pohl V, Vassart G (1985). "An unusually long poly(purine)-poly(pyrimidine) sequence is located upstream from the human thyroglobulin gene". Nucleic Acids Res. 13 (14): 5127–44. doi:10.1093/nar/13.14.5127. PMC 321854
 . PMID 2991855.
. PMID 2991855. - Baas F, van Ommen GJ, Bikker H, Arnberg AC, de Vijlder JJ (1986). "The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb". Nucleic Acids Res. 14 (13): 5171–86. doi:10.1093/nar/14.13.5171. PMC 311533
 . PMID 3016640.
. PMID 3016640. - Kubak BM, Potempa LA, Anderson B, Mahklouf S, Venegas M, Gewurz H, Gewurz AT (1989). "Evidence that serum amyloid P component binds to mannose-terminated sequences of polysaccharides and glycoproteins". Mol. Immunol. 25 (9): 851–8. doi:10.1016/0161-5890(88)90121-6. PMID 3211159.
- Malthiéry Y, Lissitzky S (1987). "Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA". Eur. J. Biochem. 165 (3): 491–8. doi:10.1111/j.1432-1033.1987.tb11466.x. PMID 3595599.
- Parma J, Christophe D, Pohl V, Vassart G (1988). "Structural organization of the 5' region of the thyroglobulin gene. Evidence for intron loss and "exonization" during evolution". J. Mol. Biol. 196 (4): 769–79. doi:10.1016/0022-2836(87)90403-7. PMID 3681978.
- Bergé-Lefranc JL, Cartouzou G, Mattéi MG, Passage E, Malezet-Desmoulins C, Lissitzky S (1985). "Localization of the thyroglobulin gene by in situ hybridization to human chromosomes". Hum. Genet. 69 (1): 28–31. doi:10.1007/BF00295525. PMID 3967888.
- Malthiéry Y, Lissitzky S (1985). "Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence". Eur. J. Biochem. 147 (1): 53–8. doi:10.1111/j.1432-1033.1985.tb08717.x. PMID 3971976.
- Avvedimento VE, Di Lauro R, Monticelli A, Bernardi F, Patracchini P, Calzolari E, Martini G, Varrone S (1985). "Mapping of human thyroglobulin gene on the long arm of chromosome 8 by in situ hybridization". Hum. Genet. 71 (2): 163–6. doi:10.1007/BF00283375. PMID 4043966.
- Xiao S, Pollock HG, Taurog A, Rawitch AB (1995). "Characterization of hormonogenic sites in an N-terminal, cyanogen bromide fragment of human thyroglobulin". Arch. Biochem. Biophys. 320 (1): 96–105. doi:10.1006/abbi.1995.1346. PMID 7793989.
- Corral J, Martín C, Pérez R, Sánchez I, Mories MT, San Millan JL, Miralles JM, González-Sarmiento R (1993). "Thyroglobulin gene point mutation associated with non-endemic simple goitre". Lancet. 341 (8843): 462–4. doi:10.1016/0140-6736(93)90209-Y. PMID 8094490.
- Gentile F, Salvatore G (1994). "Preferential sites of proteolytic cleavage of bovine, human and rat thyroglobulin. The use of limited proteolysis to detect solvent-exposed regions of the primary structure". Eur. J. Biochem. 218 (2): 603–21. doi:10.1111/j.1432-1033.1993.tb18414.x. PMID 8269951.
- Mallet B, Lejeune PJ, Baudry N, Niccoli P, Carayon P, Franc JL (1996). "N-glycans modulate in vivo and in vitro thyroid hormone synthesis. Study at the N-terminal domain of thyroglobulin". J. Biol. Chem. 270 (50): 29881–8. doi:10.1074/jbc.270.50.29881. PMID 8530385.
- Yang SX, Pollock HG, Rawitch AB (1996). "Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin". Arch. Biochem. Biophys. 327 (1): 61–70. doi:10.1006/abbi.1996.0093. PMID 8615697.
- Molina F, Bouanani M, Pau B, Granier C (1996). "Characterization of the type-1 repeat from thyroglobulin, a cysteine-rich module found in proteins from different families". Eur. J. Biochem. 240 (1): 125–33. doi:10.1111/j.1432-1033.1996.0125h.x. PMID 8797845.
- Grani G, Fumarola A (Jun 2014). "Thyroglobulin in Lymph Node Fine-Needle Aspiration Washout: A Systematic Review and Meta-analysis of Diagnostic Accuracy.". The Journal of Clinical Endocrinology and Metabolism. 99 (6): 1970–82. doi:10.1210/jc.2014-1098. PMID 24617715.
External links
- Thyroglobulin - Lab Tests Online
- Histology at KUMC endo-endo11
- Overview at colostate.edu
- Histology image: 14302loa – Histology Learning System at Boston University