Thrips
| Thrips Temporal range: 299–0 Ma Permian – recent | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Subclass: | Pterygota |
| Superorder: | Exopterygota |
| Order: | Thysanoptera Haliday, 1836 |
| Families | |
|
Terebrantia
| |
Thrips (order Thysanoptera) are minute, slender insects with fringed wings (thus the scientific name, from the Greek θύσανος thysanos ("fringe") + πτερόν pteron ("wing")).[1] Other common names for thrips include thunderflies, thunderbugs, storm flies, thunderblights, storm bugs, corn flies, corn lice and physopods. Thrips species feed on a large variety of plants and animals by puncturing them and sucking up the contents. A large number of thrips species are considered pests, because they feed on plants with commercial value. Some species of thrips feed on other insects or mites and are considered beneficial, while some feed on fungal spores or pollen. Approximately 6,000 species have been described. Thrips are generally tiny (1 mm long or less) and are not good flyers, although they can be carried long distances by the wind. In the right conditions, like indoor growing rooms or greenhouses, many species can exponentially increase in population size and form large swarms because of a lack of natural predators, making them an irritation to humans.
Like the words sheep, deer, and moose, the word thrips is used for both the singular and plural forms, so there may be many thrips or a single thrips.[2] The word thrips is from the Greek θρίψ, meaning "woodworm".[2][3]
Morphology and classification
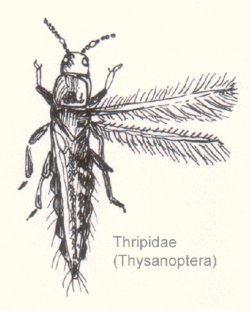
Characteristics
Thrips are small hemimetabolic insects with a distinctive cigar-shaped bauplan. They are elongated with transversely constricted bodies. They range in size from 0.5 to 14 millimetres (0.020 to 0.551 in) in length for the larger predatory thrips, but most thrips are about 1 mm in length. Flight-capable thrips have two similar, strap-like pairs of wings with a ciliated fringe (from which the order derives its name). Their legs usually end in two tarsal segments with a bladder-like structure known as an "arolium" at the pretarsus. This structure can be everted by means of hemolymph pressure, enabling the insect to walk on vertical surfaces.[4][5]
Thrips have asymmetrical mouthparts that are also unique to the group. Unlike the Hemiptera, the right mandible of thrips is reduced and vestigial – and in some species completely absent. The left mandible is larger, and forms a narrow stylet used to pierce the cell wall of tissues.[6] Some species may then inject digestive enzymes as the maxillary stylets and hypopharynx are inserted into the opening to drain cellular fluids.[7][8] This process leaves a distinctive silvery or bronze scarring on the surfaces of the stems or leaves where the thrips feed.[9]
Thysanoptera is divided into two suborders: Terebrantia, and Tubulifera; these can be distinguished by morphological, behavioral, and developmental characteristics. Members of Tubulifera can be identified by their characteristic tube-shaped apical abdominal segment, egg-laying atop the surface of leaves, and three "pupal" stages. Females of the eight families of the Terebrantia all possess the eponymous saw-like ovipositor on the anteapical abdominal segment, lay eggs singly within plant tissue, and have two "pupal" stages.
Evolution and systematics
The Thysanoptera were first described in 1744 as a genus Physapus by De Geer, and then renamed Thrips by Linnaeus in 1758. In 1836 Haliday promoted the genus to the taxonomic rank of order, renaming them Thysanoptera. There are currently over six thousand species of thrips recognized, grouped into 777 extant genera and sixty fossil genera.[10]
The earliest fossils of thrips date back to Permian (Permothrips longipennis Martynov, 1935). By the Early Cretaceous, true thrips became much more abundant.[11] The extant family Merothripidae most resemble these ancestral Thysanoptera, and are probably basal to the order.[12]
These families are currently (2013) recognized:[13][14]
- Suborder Terebrantia
- Adiheterothripidae Shumsher, 1946 (11 genera)
- Aeolothripidae Uzel, 1895 (29 genera) – banded thrips and broad-winged thrips
- Fauriellidae Priesner, 1949 (four genera)
- †Hemithripidae Bagnall, 1923 (one fossil genus, Hemithrips with 15 species)
- Heterothripidae Bagnall, 1912 (seven genera)
- † Jezzinothripidae zur Strassen, 1973 (included by some authors in Merothripidae)
- †Karataothripidae Sharov, 1972 (one fossil species, Karataothrips jurassicus)
- Melanthripidae Bagnall, 1913 (six genera)
- Merothripidae Hood, 1914 (five genera) – large-legged thrips
- † Scudderothripidae zur Strassen, 1973 (included by some authors in Stenurothripidae)
- Thripidae Stevens, 1829 (292 genera in four subfamilies) – common thrips
- † Triassothripidae Grimaldi & Shmakov, 2004 (two fossil genera)
- Uzelothripidae Hood, 1952 (one species, Uzelothrips scabrosus)
- Suborder Tubulifera
- Phlaeothripidae (447 genera in two subfamilies)
Bhatti provides an alternative classification based on morphological characters that would split the group into two orders and forty different families.[15][16][17] This classification has been less accepted by taxonomists as it can be derailed by convergent evolution and does not follow phylogenetic principles of classification.
Ecology
Natural history
Thrips are believed to have descended from a mycetophilic (fungus-feeding) ancestor during the Mesozoic,[11] and many groups still feed upon and inadvertently redistribute fungal spores, but most research has focused on those species feeding on or in association with economically significant crops. Some thrips are predatory, but the majority are phytophagous insects feeding on pollen and the chloroplasts harvested from the outer layer of plant epidermal and mesophyll cells.[9][18] These species are minute organisms that prefer to feed within the tightly packed apical buds of new growth. Feeding usually occurs along the main vein or ribs of leaves and petals.[19][20]
Flower-feeding thrips may be responsible for pollination while feeding,[21][22][23][24][25] but their most obvious contribution to their ecosystem remains the damage they can cause during feeding. This impact may fall across a broad selection of prey items, as there is considerable breadth in host affinity across the order, and even within a species, varying degrees of fidelity to a described host remain.[18][26] Family Thripidae is particularly notorious for members with broad host ranges, and the majority of pest thrips come from this family.[27][28]
While poorly documented, chemical communication is believed to be important to the group.[29] Anal secretions are produced in the hindgut,[30] and released along the posterior setae as predator deterrents.[19][30][31] Many thrips form galls on plants when feeding or laying their eggs. Some of the gall-forming Phlaeothripidae, such as genera Kladothrips[32] and Oncothrips[33] form eusocial groups similar to ant colonies, with reproductive queens and nonreproductive soldier castes.[34][35][36]
Lifecycle
The rate at which thrips move through their developmental cycles is highly dependent upon environmental conditions, including the temperature and nutrient quality of their food sources. Thrips begin their lives as eggs. These are extremely small (about 0.2 mm long) and kidney-shaped. Hatching may take from as little as a day to several weeks. The females of the suborder Terebrantia are equipped with an ovipositor, which they use to cut slits in plant tissue and then insert their eggs, one per slit. Females of the suborder Tubulifera lack an ovipositor and lay their eggs singly or in small groups on the outside surfaces of plants. Thrips then pass through two wingless instars of nymph.

As hemimetabolous insects, the Thysanoptera do not actually undergo complete metamorphosis, but pass through a similar stage in which they do not feed and are mostly immobile. Both suborders of thrips will first enter a short prepupal stage lasting a day at most, during which they will seek out dark crevices on plants, hiding in the tightly packed flower buds or bark – or drop off of the plant entirely, burrowing into leaf litter or loose soil. Some thrips will then construct a pupal cell or cocoon. In Terebrantian thrips, a single pupal instar follows, whereas in the Tubulifera, two pupal stages will follow. During these stages, wing-buds and reproductive structures will grow and mature into their adult forms.
All described genera of thrips are haplodiploid organisms capable of parthenogenesis, with some favoring arrhenotoky and others displaying thelytoky.[19][20][37] In some cases the sex-determining bacterial endosymbiont Wolbachia plays a role in defining the reproductive mode for some populations of thrips.[26][37][38] Several normally bisexual species have become established in the United States with only members of a single sex present.[19][20][37][39]
When mating occurs, it may last from minutes to hours. Most female thrips have a preoviposition period lasting from a day to a week during which their eggs mature, and before which they cannot mate.
Human impact

Many thrips are pests of commercial crops due to the damage caused by feeding on developing flowers or vegetables, causing discoloration, deformities, and reduced marketability of the crop. Thrips may also serve as vectors for plant diseases, such as Tospoviruses.[40] Over 20 plant-infecting viruses are known to be transmitted by thrips. These enveloped viruses are considered among some of the most damaging of emerging plant pathogens around the world. Virus members include the tomato spotted wilt virus and the impatiens necrotic spot viruses. The western flower thrips, Frankliniella occidentalis, now has a worldwide distribution and is considered the primary vector of plant diseases caused by Tospoviruses.
This global explosion in thrips species' range is not uncommon,[41] as their small sizes and predisposition towards enclosed places makes them difficult to detect by phytosanitary inspection. When coupled with the increasing globalization of trade and the growth of greenhouse agriculture, thrips, unsurprisingly, are among the fastest growing group of invasive species in the world. Examples include the aforementioned Frankliniella occidentalis, Scirtothrips dorsalis, and Thrips palmi.
Thrips develop resistance to pesticides easily and there is constant research on how to control them. This makes thrips ideal as models for testing the effectiveness of new pesticides and methods. [42]
Flower-feeding thrips are routinely attracted to bright floral colors (including white, blue, and especially yellow), and will land and attempt to feed. It is not uncommon for some species (e.g., Frankliniella tritici and Limothrips cerealium) to "bite" humans under such circumstances. Although no species feed on blood and no known animal disease is transmitted by thrips, some skin irritation has been described.[43]
Although many thrips are considered to be pests, they have been found to aid in pollination of plants as well. It has been found that a species of thrips Thrips setipennis is the sole pollinator of Wilkiea huegeliana, which is a small, uni-sexual annually flowering tree or shrub that is found in the rainforests of eastern Australia. In Australia, T. setipennis serves as an obligate pollinator for other rainforest plant species, including Rapanea howittiana and Rapanea variabilis. [44] Thrips have also been found to be the primary pollinators of plants in the family Ericaceae[45]
Management
Due to their small sizes and high rates of reproduction, thrips are difficult to control using classical biological control. All predators must be small and slender enough to penetrate the crevices where thrips hide while feeding, and then prey extensively on eggs and larvae. Only two families of parasitoid Hymenoptera are known to parasitize eggs and larvae, the Eulophidae and the Trichogrammatidae. Other biocontrol agents of adults and larvae include aphid wasps, anthocorid bugs of genus Orius, and phytoseiid mites. For this reason, many growers are occasionally forced to make limited use of pesticides to control thrips populations in the field and in greenhouses. Another effective strategy for pest thrips are biological insecticides, including Beauveria bassiana or Verticillium lecanii. These demonstrate a clear effect on eggs, larvae and adults of thrips. Insecticidal soap spray is effective against thrips. It is commercially available or can be made of certain types of household soap. Scientists in Japan report that significant reductions in larva and adult melon thripes occur when plants are illuminated with red light. [46]
References
- ↑ Tipping, C. (2008). Thrips (Thysanoptera). Pages 3769–3771 in Encyclopedia of Entomology, John L. Capinera, ed. Springer, New York
- 1 2 Kobro, Sverre (2011). "Checklist of Nordic Thysanoptera" (PDF). Norwegian Journal of Entomology. 58: 21–26. Retrieved October 25, 2014.
- ↑ W. D. J. Kirk (1996). Thrips: Naturalists' Handbooks 25. The Richmond Publishing Company.
- ↑ Gillott, Cedric (2005). Entomology. Springer. p. 234. ISBN 0-306-44967-6.
- ↑ Heming, BS (1971). Functional morphology of the thysanopteran pretarsus. Canadian Journal of Zoology. 49: 91–108.
- ↑ Childers, C. C., and D. S. Achor. 1989. Structure of the mouthparts of Frankliniella bispinosa (Morgan) (Thysanoptera: Thripidae). In B. L. Parker, M. Skinner and T. Lewis [eds.], Towards Understanding Thysanoptera. Proceedings of the International Conference on Thrips. USDA Technical Report NE-147, Radnor, PA.
- ↑ W. B. Hunter & D. E. Ullman (1989). "Analysis of mouthpart movements during feeding of Frankliniella occidentalis (Pergande) and F. schultzei Trybom (Thysanoptera: Thripidae)". International Journal of Insect Morphology and Embryology 18: 161–171. doi:10.1016/0020-7322(89)90024-X.
- ↑ W. B. Hunter, D. E. Ullman & A. Moore (1994). "Electronic monitoring: characterizing the feeding behavior of western flower thrips (Thysanoptera: Thripidae)". in M. M. Ellsbury, E. A. Backus & D. L. Ullman. History, Development, and Application of AC Electronic Insect Feeding Monitors. Thomas Say Publications in Entomology. pp. 73–85.
- 1 2 Heming, B. S. 1993. Structure, function, ontogeny, and evolution of feeding in thrips (Thysanoptera). In C. W. Shaefer and R. A. B. Leschen [eds.], Functional Morphology of Insect Feeding. Entomological Society of America, Lanham, Maryland.
- ↑ http://thrips.info/wiki/Main_Page Thrips Wiki, retrieved on 21 MAY 2015
- 1 2 D.Grimaldi,A.Shmakov, N.Fraser, Mesozoic Thrips and Early Evolution of the Order Thysanoptera (Insecta).Journal of Paleontology, Sept. 2004
- ↑ Mound, L. A. 1997. Biological diversity., pp. 197–215. In T. Lewis [ed.], Thrips As Crop Pests. CAB International, Wallingford, UK.
- ↑ Buckman RS, Mound LA & Whiting MF (2013) Phylogeny of thrips (Insecta: Thysanoptera) based on five molecular loci. Systematic Entomology 38: 123-133
- ↑ Mound LA 2011. Order Thysanoptera Haliday, 1836. Pp 201–202 In Zhang, Z.-Q. [Ed] Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148, 1–237
- ↑ Bhatti JS (1994) Phylogenetic relationships among Thysanoptera (Insecta) with particular reference to the families of the Order Tubulifera. Zoology (Journal of Pure and Applied Zoology) 4 (1993): 93-130
- ↑ Bhatti JS (1998) New structural features in the Order Tubulifera (Insecta). 1. Amalgamation of labro-maxillary complex with cranium and other cephalic structures. Zoology (Journal of Pure and Applied Zoology) 5: 147-176
- ↑ Bhatti JS (2006) The classification of Terebrantia (Insecta) into families. Oriental Insects 40: 339-375
- 1 2 Kirk, W. D. J. 1995. Feeding behavior and nutritional requirements, pp. 21 – 29. In B. L. Parker, M. Skinner and T. Lewis [eds.], Thrips Biology and Management. Plenum Press, New York, NY.
- 1 2 3 4 Lewis, T. 1973. Thrips. Their biology, ecology and economic importance. Academic Press, London, GB.
- 1 2 3 Lewis, T. 1997. Thrips as crop pests. CAB International, Oxon, GB.
- ↑ Darwin, C. (1892) The effects of cross and self fertilization in the vegetable kingdom. D. Appleton & Company, New York.
- ↑ Williams G, Adams P & Mound LA (2001) Thrips (Thysanoptera) pollination in Australian subtropical rainforests, with particular reference to pollination of Wilkiea huegeliana. (Monimiaceae). Journal of Natural History 35: 1-21
- ↑ Sakai S (September 2001). "Thrips pollination of androdioecious Castilla elastica (Moraceae) in a seasonal tropical forest". American Journal of Botany. 88 (9): 1527–34. doi:10.2307/3558396. JSTOR 3558396. PMID 21669685.
- ↑ Saxena, P., M. R. Vijayaraghavan, R. K. Sarbhoy, and U. Raizada. 1996. Pollination and gene flow in chillies with Scirtothrips dorsalis as pollen vectors. Phytomorphology 46: 317 – 327.
- ↑ Frame, Dawn (November 2003). "Generalist Flowers, Biodiversity and Florivory: Implications for Angiosperm Origins". Taxon. 52 (4): 681–5. doi:10.2307/3647343. JSTOR 3647343.
- 1 2 Mound, L. A. 2005. Thysanoptera: diversity and interactions. Annual Review of Entomology 50: 247 – 269.
- ↑ Bailey, S. F. 1940. The distribution of injurious thrips in the United States. Journal of Economic Entomology 33: 133 – 136.
- ↑ Ananthakrishnan TN (1993). "Bionomics of Thrips". Annual Review of Entomology. 38: 71–92. doi:10.1146/annurev.en.38.010193.000443.
- ↑ Blum, M. S. 1991. Chemical ecology of the Thysanoptera, pp. 95 – 108. In B. L. Parker, M. Skinner and T. Lewis [eds.], Towards Understanding Thysanoptera. Proceedings of the International Conference on Thrips. USDA Technical Report NE-147, Radnor, PA.
- 1 2 Howard, D. F., M. S. Blum, and H. M. Fales. 1983. Defense in thrips: forbidding fruitiness of a lactone. Science 220: 335 – 336.
- ↑ Tschuch, G., P. Lindemann, and G. Moritz. 2002. Chemical defence in thrips, pp. 277 – 278. In L. A. Mound and R. Marullo [eds.], Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera. CSIRO Entomology, Reggio Calabria, Italy.
- ↑ Kranz, BD; Schwarz, MP; Mound, LA; Crespi, BJ (1999). Social biology and sex ratios of the eusocial gall-inducing thrips Kladothrips hamiltoni. Ecological Entomology, 24: 432 - 442.
- ↑ Kranz, Brenda D.; Schwarz, Michael P.; Wills, Taryn E.; Chapman, Thomas W.; Morris, David C.; Crespi, Bernard J. (July 2001). "A Fully Reproductive Fighting Morph in a Soldier Clade of Gall-Inducing Thrips (Oncothrips morrisi)". Behavioral Ecology and Sociobiology. 50 (2): 151–61. doi:10.1007/s002650100347. JSTOR 4601948.
- ↑ Crespi, BJ; Mound, LA (1997). Ecology and evolution of social behaviour among Australian gall thrips and their allies. In: Choe, JC; Crespi, BJ (eds): The evolution of social behaviour of insects and arachnids. Cambridge: Cambridge University Press; 166 – 180.
- ↑ Chapman, TW; Crespi, BJ; (1998). High relatedness and inbreeding in two species of haplodiploid eusocial thrips (Insecta: Thysanoptera) revealed by microsatellite analysis. Behavioral Ecology and Sociobiology. 43: 301 - 306.
- ↑ Kranz, Brenda D.; Schwarz, Michael P.; Morris, David C.; Crespi, Bernard J. (February 2002). "Life history of Kladothrips ellobus and Oncothrips rodwayi: insight into the origin and loss of soldiers in gall-inducing thrips". Ecological Entomology. 27 (1): 49–57. doi:10.1046/j.1365-2311.2002.0380a.x.
- 1 2 3 EVOLUTION OF ASEXUALITY VIA DIFFERENT MECHANISMS IN GRASS THRIPS (THYSANOPTERA: Aptinothrips) van der Kooi, CJ & Schwander, T (2014) Evolution 68: 1883-1893
- ↑ Kumm S, Moritz G (December 2008). "First detection of Wolbachia in arrhenotokous populations of thrips species (Thysanoptera: Thripidae and Phlaeothripidae) and its role in reproduction". Environmental Entomology. 37 (6): 1422–8. doi:10.1603/0046-225X-37.6.1422. PMID 19161685.
- ↑ Stannard, L. J. 1968. The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey 21: 215 – 552.
- ↑ L. R. Nault (1997). "Arthropod transmission of plant viruses: a new synthesis". Annals of Entomological Society of America 90: 521–541.
- ↑ Morse, JG; Hoddle, MS (2005). Invasion biology of thrips. Annual Review of Entomology 51: 67 – 89.
- ↑ Kivett, Jessica M.; Cloyd, Raymond A.; Bello, Nora M. (2015-08-01). "Insecticide Rotation Programs with Entomopathogenic Organisms for Suppression of Western Flower Thrips (Thysanoptera: Thripidae) Adult Populations under Greenhouse Conditions". Journal of Economic Entomology. 108 (4): 1936–1946. doi:10.1093/jee/tov155. ISSN 0022-0493. PMID 26470338.
- ↑ Childers CC, Beshear RJ, Frantz G, Nelms M (2005) A review of thrips species biting man including records in Florida and Georgia between 1986–1997. Florida Entomologist: Vol. 88, No. 4 pp. 447–451
- ↑ Williams, G. A.; Adam, P.; Mound, L. A. (2001-01-01). "Thrips (Thysanoptera) pollination in Australian subtropical rainforests, with particular reference to pollination of Wilkiea huegeliana (Monimiaceae)". Journal of Natural History. 35 (1): 1–21. doi:10.1080/002229301447853. ISSN 0022-2933.
- ↑ García‐Fayos, Patricio; Goldarazena, Arturo (2008-07-01). "The Role of Thrips in Pollination of Arctostaphyllos uva‐ursi". International Journal of Plant Sciences. 169 (6): 776–781. doi:10.1086/588068. ISSN 1058-5893.
- ↑ KATAI, Yusuke; ISHIKAWA, Ryusuke; DOI, Makoto; MASUI, Shinichi (2015-02-01). "Efficacy of Red LED Irradiation for Controlling Thrips palmi in Greenhouse Melon Cultivation.". Japanese Journal of Applied Entomology & Zoology. 59 (1): 1–6. doi:10.1303/jjaez.2015.1.}
External links
| Wikimedia Commons has media related to Thysanoptera. |
| Wikispecies has information related to: Thysanoptera |
- Thrips of the World checklist
- thrips species wiki
- Thrips images from the "Pests and Diseases Image Library (PaDIL)" of Australia
- University of California Pest Management Guidelines for Thrips
- University of California Thrips Identification
- CISR: Center for Invasive Species Research Fact Sheets
- Thrips links on the UF / IFAS Featured Creatures Web site
- Frankliniella schultzei, common blossom thrips (Thripidae)
- Gynaikothrips ficorum, Cuban laurel thrips (Phlaeothripidae)
- Heliothrips haemorrhoidalis , greenhouse thrips (Thripidae)
- Scirtothrips dorsalis, chilli thrips (Thripidae)
- Selenothrips rubrocinctus, redbanded thrips (Thripidae)
- Thrips palmi, melon thrips (Thripidae)
- Thrips simplex, gladiolus thrips (Thripidae)

