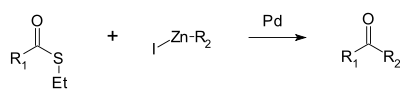Thioester
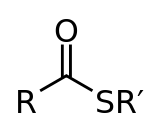
In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.[1]
Synthesis
The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol:[1]
- RSNa + R′COCl → R′COSR + NaCl
Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example thioacetate esters are commonly prepared by alkylation of potassium thioacetate:[1]
- CH3COSK + RX → CH3COSR + KCl
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using Mannich bases and the thiocarboxylic acid:
- CH3COSH + R′2NCH2OH → CH3COSCH2NR′2 + H2O
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of dehydrating agents:[2][3]
- RSH + R′CO2H → RSC(O)R′ + H2O
A typical dehydration agent is DCC.[4] Acid anhydrides and some lactones also give thioesters upon treatment with thiols in the presence of a base.
Thioesters can be conveniently prepared from alcohols by the Mitsunobu reaction, using thioacetic acid.[5]
They also arise via carbonylation of alkynes and alkenes in the presence of thiols.[6]
Reactions
The carbonyl center in thioesters is reactive toward nucleophiles, even water. Thus, thioesters are common intermediates in the conversion of alkyl halides to alkyl thiols.Thioesters and amines combine to give amides:
Thioesters provide useful chemoselectivity in the synthesis of biomolecules.[7]
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
The C-H groups adjacent to the carbonyl in thioesters are mildly acidic (more so than in esters[8][9]) and undergo aldol condensations. This kind of reaction occurs in the biosynthesis of fatty acids.
Biochemistry

Thioesters are common intermediates in many biosynthetic reactions, including the formation and degradation of fatty acids and mevalonate, precursor to steroids. Examples include malonyl-CoA, acetoacetyl-CoA, propionyl-CoA, and cinnamoyl-CoA. Acetogenesis proceeds via the formation of acetyl-CoA. The biosynthesis of lignin, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of caffeic acid.[10] These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with ubiquitin, which tags the protein for degradation.
Oxidation of the sulfur atom in thioesters (thiolactones) is postulated in the bioactivation of the antithrombotic prodrugs ticlopidine, clopidogrel, and prasugrel.[11][12]
Thioesters and the origin of life
As posited in a "Thioester World", thioesters are possible precursors to life.[13] As de Duve explains:
It is revealing that thioesters are obligatory intermediates in several key processes in which ATP is either used or regenerated. Thioesters are involved in the synthesis of all esters, including those found in complex lipids. They also participate in the synthesis of a number of other cellular components, including peptides, fatty acids, sterols, terpenes, porphyrins, and others. In addition, thioesters are formed as key intermediates in several particularly ancient processes that result in the assembly of ATP. In both these instances, the thioester is closer than ATP to the process that uses or yields energy. In other words, thioesters could have actually played the role of ATP in a "thioester world" initially devoid of ATP. Eventually, [these] thioesters could have served to usher in ATP through its ability to support the formation of bonds between phosphate groups.
However, due to the high free energy change of thioester's hydrolysis and corresponding their low equilibrium constants, it is unlikely that these compounds could have accumulated abiotically to any significant extant especially in hydrothermal vent conditions.[14]
Isomeric compounds: thionoesters

Thionoesters are isomeric with thioesters. In a thionoester, sulfur replaces the carbonyl oxygen in an ester. Methyl thionobenzoate is C6H5C(S)OCH3. Such compounds are typically prepared by the reaction of the thioacyl chloride with an alcohol, but they can also be made by the reaction of Lawesson's reagent with esters.[15]

See also
References
- 1 2 3 Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. doi:10.1002/9780470771099.ch15
- ↑ Fujiwara, S.; Kambe, N. (2005). "Thio-, Seleno-, and Telluro-Carboxylic Acid Esters". Topics in Current Chemistry. 251. Berlin / Heidelberg: Springer. pp. 87–140. doi:10.1007/b101007. ISBN 978-3-540-23012-0.
- ↑ "Synthesis of thioesters". Organic Chemistry Portal.
- ↑ Mori, Y.; Seki, M. (2007). "Synthesis of Multifunctionalized Ketones Through the Fukuyama Coupling Reaction Catalyzed by Pearlman's Catalyst: Preparation of Ethyl 6-oxotridecanoate". Org. Synth. 84: 285.; Coll. Vol., 11, p. 281
- ↑ Volante, R. (1981). "A new, highly efficient method for the conversion of alcohols to thiolesters and thiols". Tetrahedron Letters. 22 (33): 3119–3122. doi:10.1016/S0040-4039(01)81842-6.
- ↑ "Carbonylation", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, 2005, doi:10.1002/14356007.a05_217.pub2
|first1=missing|last1=in Authors list (help) - ↑ McGrath, N. A.; Raines, R. T. (2011). "Chemoselectivity in chemical biology: Acyl transfer reactions with sulfur and selenium". Acc. Chem. Res. 44 (9): 752–761. doi:10.1021/ar200081s. PMC 3242736
 . PMID 21639109.
. PMID 21639109. - ↑ Bew, S. P.; Stephenson, G. R.; Rouden, J.; Martinez-Lozano, L.A.; Seylani, H. (2013). "Malonic Acid Half Oxyesters and Thioesters: Solvent-Free Synthesis and DFT Analysis of Their Enols". Org. Lett. 15 (15): 3805–3807. doi:10.1021/ol400804b.
- ↑ Bordwell, F. G.; Fried, H. E. (1991). "Heterocyclic Aromatic Anions with 4n + 2 pi-Electrons". J. Org. Chem. 56 (13): 4218–4223. doi:10.1021/jo00013a027.
- ↑ Lehninger, A. L.; Nelson, D. L.; Cox, M. M. (2000). Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6.
- ↑ Mansuy, D.; Dansette, P. M. (2011). "Sulfenic acids as reactive intermediates in xenobiotic metabolism". Archives of Biochemistry and Biophysics. 507 (1): 174–185. doi:10.1016/j.abb.2010.09.015. PMID 20869346.
- ↑ Dansette, P. M.; Rosi, J.; Debernardi, J.; Bertho, G.; Mansuy, D. (2012). "Metabolic Activation of Prasugrel: Nature of the Two Competitive Pathways Resulting in the Opening of Its Thiophene Ring". Chemical Research in Toxicology. 25 (5): 1058–1065. doi:10.1021/tx3000279.
- ↑ de Duve, C. (1995). "The Beginnings of Life on Earth". American Scientist. 83 (5): 428–437. Bibcode:1995AmSci..83..428M.
- ↑ Chandru, Kuhan; Gilbert, Alexis; Butch, Christopher; Aono, Masashi; Cleaves, Henderson James II (21 July 2016). "The Abiotic Chemistry of Thiolated Acetate Derivatives and the Origin of Life". Scientific Reports. 6 (29883). doi:10.1038/srep29883.
- ↑ Cremlyn, R. J. (1996). An Introduction to Organosulfur Chemistry. Chichester: John Wiley and Sons. ISBN 0-471-95512-4.