Tellurite (ion)
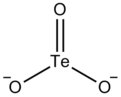 | |
| Names | |
|---|---|
| Systematic IUPAC name | |
| Identifiers | |
| 15852-22-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30477 |
| ChemSpider | 102958 |
| 100741 | |
| PubChem | 115037 |
| |
| |
| Properties | |
| O3Te2− | |
| Molar mass | 175.6 g mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The tellurite ion is TeO2−
3. A tellurite (compound), for example sodium tellurite, is a compound that contains this ion. Tellurites are highly stable tellurium compounds, although they can be reduced to elemental tellurium by electrolysis or a strong reducing agent.
Acidified forms
In slightly acidic conditions, hydrogen tellurite ion, HTeO−
3, is prevalent; with more acidic conditions tellurous acid, H2TeO3, is prevalent. Only in basic conditions is tellurite formed.
Preparation
Most tellurite compounds can be formed by heating the relevant oxide with tellurium dioxide, e.g., Na2O + TeO2 → Na2TeO3.
Uses
Potassium tellurite (K2TeO3) is used together with agar as part of a selective medium for growth of some bacteria (Clauberg medium). Corynebacteria and some other species reduce TeO2−
3 to elemental Te, which stains the bacteria black.
See also
- List of tellurites
References
- ↑ "Tellurous Acid - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ "Tellurite (CHEBI:30477)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.