Technetium (99mTc) etarfolatide
 | |
| Clinical data | |
|---|---|
| ATC code | V09IA08 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
479410-20-3 |
| PubChem (CID) | 71300440 |
| UNII |
WVU7R99FQ6 |
| Chemical and physical data | |
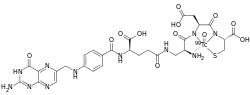
| Formula | C29H32N11O12S99mTc |
| Molar mass | 855.595 g/mol |
| 3D model (Jmol) | Interactive image |
| |
Technetium (99mTc) etarfolatide is an investigational non-invasive, folate receptor-targeting companion imaging agent that is being developed by Endocyte.[1] Etarfolatide consists of a small molecule targeting the folate receptor and an imaging agent, which is based on technetium-99m. This companion imaging agent identifies cells expressing the folate receptor, including cancer and inflammatory cells.[2]
Etarfolatide is currently being investigated together with the corresponding small molecule drug conjugate (SMDC) vintafolide in a Phase 3 study in platinum-resistant ovarian cancer and in a Phase 2b study in non-small cell lung cancer. It identifies patients with metastases that are positive for the folate receptor and therefore more likely to respond to treatment with vintafolide.[1][2] Other folate receptor targeting SMDCs for the treatment of cancer, inflammatory diseases and kidney disease are in preclinical development and will also utilize etarfolatide as companion imaging agent.[3][4]
The European Medicines Agency (EMA) is reviewing the Marketing Authorization Application (MAA) filings for both vintafolide and etarfolatide, for the treatment of patients with folate receptor-positive platinum-resistant ovarian cancer in combination with pegylated liposomal doxorubicin (PLD).[5]
References
- 1 2 http://www.fiercemedicaldevices.com/story/endocytes-dx-spots-ovarian-and-lung-cancer-patients-ripe-merck-drug/2013-03-03
- 1 2 http://www.radiologytoday.net/archive/rt0213p12.shtml
- ↑ http://health.universityofcalifornia.edu/2012/08/02/new-drug-shows-promise-for-kidney-disease/
- ↑ http://www.insideindianabusiness.com/life-sciences.asp?ID=3298&Detail=True
- ↑ http://www.businessweek.com/ap/2012-11-27/eu-reviews-cancer-drug-from-merck-and-endocyte