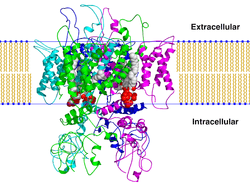
TRPV1
| View/Edit Human | View/Edit Mouse |
The transient receptor potential cation channel subfamily V member 1 (TrpV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group.[4][5] This protein is a member of the TRPV group of transient receptor potential family of ion channels.[6]
The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception).
Function
TRPV1 is a nonselective cation channel that may be activated by a wide variety of exogenous and endogenous physical and chemical stimuli. The best-known activators of TRPV1 are: temperature greater than 43 °C (109 °F); acidic conditions; capsaicin, the irritating compound in hot chili peppers; and allyl isothiocyanate, the pungent compound in mustard and wasabi.[7] The activation of TRPV1 leads to a painful, burning sensation. Its endogenous activators include: low pH (acidic conditions), the endocannabinoid anandamide, N-oleyl-dopamine, and N-arachidonoyl-dopamine. TRPV1 receptors are found mainly in the nociceptive neurons of the peripheral nervous system, but they have also been described in many other tissues, including the central nervous system. TRPV1 is involved in the transmission and modulation of pain (nociception), as well as the integration of diverse painful stimuli.[8][9]
Sensitization
The sensitivity of TRPV1 to noxious stimuli, such as high temperatures, is not static. Upon tissue damage and the consequent inflammation, a number of inflammatory mediators, such as various prostaglandins and bradykinin, are released. These agents increase the sensitivity of nociceptors to noxious stimuli. This manifests as an increased sensitivity to painful stimuli (hyperalgesia) or pain sensation in response to non-painful stimuli (allodynia). Most sensitizing pro-inflammatory agents activate the phospholipase C pathway. Phosphorylation of TRPV1 by protein kinase C have been shown to play a role in sensitization of TRPV1. The cleavage of PIP2 by PLC-beta can result in disinhibiton of TRPV1 and, as a consequence, contribute to the sensitivity of TRPV1 to noxious stimuli.
Desensitization
Upon prolonged exposure to capsaicin, TRPV1 activity decreases, a phenomenon called desensitization. Extracellular calcium ions are required for this phenomenon, thus influx of calcium and the consequential increase of intracellular calcium mediate this effect. Various signaling pathways such as calmodulin and calcineurin, and the decrease of PIP2, have been implicated in desensitization of TRPV1. Desensitization of TRPV1 is thought to underlie the paradoxical analgesic effect of capsaicin.
Clinical significance
Peripheral nervous system
Treatment of pain is an unmet medical need costing billions of dollars every year. As a result of its involvement in nociception, TRPV1 has been a prime target for the development of novel pain reducers (analgesics). Two major strategies have been used:
Antagonists
Antagonists block TRPV1 activity, thus reducing pain. Identified antagonists include the competitive antagonist capsazepine and the non-competitive antagonist ruthenium red.[4] These agents could be useful when applied systemically.[10] Numerous TRPV1 antagonists have been developed by pharmaceutical companies. TRPV1 antagonists have shown efficacy in reducing nociception from inflammatory and neuropathic pain models in rats.[11] This provides evidence that TRPV1 is capsaicin's sole receptor.[12] In humans, drugs acting at TRPV1 receptors could be used to treat neuropathic pain associated with multiple sclerosis, chemotherapy, or amputation, as well as pain associated with the inflammatory response of damaged tissue, such as in osteoarthritis.[13]
The major roadblock for the usefulness of these drugs is their effect on body temperature (hyperthermia). The role of TRPV1 in the regulation of body temperature has emerged in the last few years. Based on a number of TRPV-selective antagonists' causing an increase in body temperature (hyperthermia), it was proposed that TRPV1 is tonically active in vivo and regulates body temperature[14] by telling the body to "cool itself down". Without these signals, the body overheats. Likewise, this explains the propensity of capsaicin (a TRPV1 agonist) to cause sweating (i.e.: a signal to reduce body temperature). In a recent report, it was found that tonically active TRPV1 channels are present in the viscera and keep an ongoing suppressive effect on body temperature.[15] Recently, it was proposed that predominant function of TRPV1 is body temperature maintenance [16] Experiments have shown that TRPV1 blockade increases body temperature in multiple species, including rodents and humans, suggesting that TRPV1 is involved in body temperature maintenance.[14] Recently, AMG 517, a highly selective TRPV1 antagonist was dropped out of clinical trials due to the undesirable level of hyperthermia.[17] A second molecule, SB-705498, was also evaluated in the clinic but its effect on body temperature was not reported.[18] Recently, it was disclosed that clinical trials of two more TRPV1 antagonists, GRC 6211 and NGD 8243, have been stopped. Post translational modification of TRPV1 protein by its phosphorylation is critical for its functionality. Recent reports published from NIH suggest that Cdk5-mediated phosphorylation of TRPV1 is required for its ligand-induced channel opening.[19]
Agonists
Agonists such as capsaicin and resiniferatoxin activate TRPV1 and, upon prolonged application, cause TRPV1 activity to decrease (desensitization), leading to alleviation of pain via the subsequent decrease in the TRPV1 mediated release of inflammatory molecules following exposures to noxious stimuli. Agonists can be applied locally to the painful area in various forms, generally as a patch or an ointment. Numerous capsaicin-containing creams are available over the counter, containing low concentrations of capsaicin (0.025 - 0.075%). It is debated whether these preparations actually lead to TRPV1 desensitization; it is possible that they act via counter-irritation. Novel preparations containing higher capsaicin concentration (up to 10%) are under clinical trials.[20] 8% capsaicin patches have recently become available for clinical use, with supporting evidence demonstrating that a 30-minute treatment can provide up to 3 months analgesia by causing regression of TRPV1-containing neurons in the skin.[21] Currently, these treatments must be re-administered on a regular (albeit infrequent) schedule in order to maintain their analgesic effects.
Fatty acid metabolites
Certain metabolites of polyunsaturated fatty acids have been shown to stimulate cells in a TRPV1-dependent fashion. The metabolites of linoleic acid, including 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE), 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE, 9(S)-hydroxy-10(E),12(Z)-octadecadienoic acid (9(S)-HODE), 9(R)-hydroxy-10(E),12(Z)-octadecadienoic acid (9(R)-HODE), and their respective keto analogs, 13-oxoODE and 9-oxoODE (see 13-HODE and 9-HODE sections on Direct actions), activate peripheral and central mouse pain sensing neurons. Reports disagree on the potencies of these metabolites with, for example, the most potent one, 9(S)-HODE, requiring at least 10 micromoles/liter.[22] or a more physiological concentration of 10 nanomoles/liter[23] to activate TRPV1 in rodent neurons. The TRPV1-dependency of these metabolites' activities appears to reflect their direct interaction with TPRV1. Although relatively weak agonists of TRPV1 in comparison to anandamide,[22] these linoleate metabolites have been proposed to act through TRPV1 in mediating pain perception in rodents[23][24][25] and to cause injury to airway epithelial cells and thereby to contribute to asthma disease[26] in mice and therefore possibly humans. Certain arachidonic acid metabolites, including 20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid (see 20-Hydroxyeicosatetraenoic acid)[27] and 12(S)-hydroperoxy-5Z,8Z,10E,12S,14Z-eicosatetraenoic acid (12(S)-HpETE), 12(S)-hydroxy-5Z,8Z,10E,12S,14Z-eicosatetraenoic acid (12(S)-HETE (see 12-HETE), hepoxilin A3 (i.e. 8R/S-hydroxy-11,12-oxido-5Z,9E,14Z-eicosatrienoic acid) and HxB3 (i.e. 10R/S-hydroxy-11,12-oxido-5Z,8Z,14Z-eicosatrienoic acid) likewise activate TRPV1 and may thereby contribute to tactile hyperalgesia and allodynia (see Hepoxilin#Pain perception).[28][29][30]
Resolvin E1 (RvE1), RvD2 (see resolvins), neuroprotectin D1 (NPD1), and maresin 1 (Mar1) are metabolites of the omega 3 fatty acids, eicosapentaenoic acid (for RvE1) or docosahexaenoic acid (for RvD2, NPD1, and Mar1). These metabolites are members of the specialized proresolving mediators (SPMs) class of metabolites that function to resolve diverse inflammatory reactions and diseases in animal models and, it its proposed, humans. These SPMs also dampen pain perception arising from various inflammation-based causes in animal models. The mechanism behind their pain-dampening effects involves the inhibition of TRPV1, probably (in at least certain cases) by an indirect effect wherein they activate other receptors located on the neruons or nearby microglia or astrocytes. CMKLR1, GPR32, FPR2, and NMDA receptors have been proposed to be the receptors through which these SPMs operate to down-regulate TRPV1 and thereby pain perception.[31][32][33][34][35]
Fatty acid conjugates
N-Arachidonoyl dopamine, a endocannabinoid found in the human CNS, structurally similar to capsaicin, activates the TRPV1 channel with an EC50 of approximately of 50 nM.[9]
N-Oleyl-dopamine, another endogenous agonist, binds bind to human VR1 with an Ki of 36 Nm.[36]
Another endocannabinoid anandamide has also been shown to act on TRPV1 receptors.[37]
AM404—an active metabolite of paracetamol—that serves as an anandamide reuptake inhibitor and COX inhibitor also serves as a potent TRPV1 agonist.[38]
The plant-biosynthesized cannabinoid cannabidiol also shows "either direct or indirect activation" of TRPV1 receptors.[39]
Central nervous system
TRPV1 is also expressed at high levels in the central nervous system and has been proposed as a target for treatment not only of pain but also for other conditions such as anxiety.[40] Furthermore, TRPV1 appears to mediate long-term synaptic depression (LTD) in the hippocampus.[41] LTD has been linked to a decrease in the ability to make new memories, unlike its opposite long-term potentiation (LTP), which aids in memory formation. A dynamic pattern of LTD and LTP occurring at many synapses provides a code for memory formation. Long-term depression and subsequent pruning of synapses with reduced activity is an important aspect of memory formation. In rat brain slices, activation of TRPV1 with heat or capsaicin induced LTD while capsazepine blocked capsaicin's ability to induce LTD.[41] In the brainstem (solitary tract nucleus), TRPV1 controls the asynchronous and spontaneous release of glutamate from unmyelinated cranial visceral afferents - release processes that are active at normal temperatures and hence quite distinct from TRPV1 responses in painful heat.[42] Hence, there may be therapeutic potential in modulating TRPV1 in the central nervous system, perhaps as a treatment for epilepsy (TRPV1 is already a target in the peripheral nervous system for pain relief).
Interactions
TRPV1 has been shown to interact with:
Discovery
The dorsal root ganglion (DRG) neurons of mammals were known to express a heat-sensitive ion channel that could be activated by capsaicin.[45] The research group of David Julius, therefore, created a cDNA library of genes expressed in dorsal root ganglion neurons, expressed the clones in HEK 293 cells, and looked for cells that respond to capsaicin with calcium influx (which HEK-293 normally not do). After several rounds of screening and dividing the library, a single clone encoding the TRPV1 channel was finally identified in 1997. It was the first TRPV channel to be identified.[4]
See also
- Capsaicin
- Capsinoids
- Vanilloids
- Vanillotoxin
- Cannabinoid receptor
- Discovery and development of TRPV1 antagonists
- Ruthenium red
References
- ↑ "Drugs that physically interact with Transient receptor potential cation channel subfamily V member 1 view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (October 1997). "The capsaicin receptor: a heat-activated ion channel in the pain pathway". Nature. 389 (6653): 816–24. doi:10.1038/39807. PMID 9349813.
- ↑ Xue Q, Yu Y, Trilk SL, Jong BE, Schumacher MA (August 2001). "The genomic organization of the gene encoding the vanilloid receptor: evidence for multiple splice variants". Genomics. 76 (1-3): 14–20. doi:10.1006/geno.2001.6582. PMID 11549313.
- ↑ Clapham DE, Julius D, Montell C, Schultz G (December 2005). "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels". Pharmacol. Rev. 57 (4): 427–50. doi:10.1124/pr.57.4.6. PMID 16382100.
- ↑ Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K (February 2011). "The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil". Curr. Biol. 21 (4): 316–21. doi:10.1016/j.cub.2011.01.031. PMID 21315593.
- ↑ Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR (2006). "TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists". J. Neurosci. 26 (37): 9385–93. doi:10.1523/JNEUROSCI.1246-06.2006. PMID 16971522.
- 1 2 Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V (June 2002). "An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors". Proc. Natl. Acad. Sci. U.S.A. 99 (12): 8400–5. doi:10.1073/pnas.122196999. PMC 123079
 . PMID 12060783.
. PMID 12060783. - ↑ Khairatkar-Joshi N, Szallasi A (2009). "TRPV1 antagonists: the challenges for therapeutic targeting". Trends Mol Med. 15 (1): 14–22. doi:10.1016/j.molmed.2008.11.004. PMID 19097938.
- ↑ Jhaveri MD, Elmes SJ, Kendall DA, Chapman V (2005). "Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naïve, carrageenan-inflamed and neuropathic rats". Eur. J. Neurosci. 22 (2): 361–70. doi:10.1111/j.1460-9568.2005.04227.x. PMID 16045489.
- ↑ Story GM, Crus-Orengo L (2008). "Feel the Burn". American Scientist. 95 (4): 326–333. doi:10.1511/2007.66.326. ISSN 0003-0996. Archived from the original on January 19, 2008.
- ↑ Gunthorpe MJ, Szallasi A (2008). "Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms". Curr. Pharm. Des. 14 (1): 32–41. doi:10.2174/138161208783330754. PMID 18220816.
- 1 2 Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC (March 2007). "The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation". J. Neurosci. 27 (13): 3366–74. doi:10.1523/JNEUROSCI.4833-06.2007. PMID 17392452.
- ↑ Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA (July 2007). "Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors". J. Neurosci. 27 (28): 7459–68. doi:10.1523/JNEUROSCI.1483-07.2007. PMID 17626206.
- ↑ Gavva NR (2008). "Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1". Trends Pharmacol. Sci. 29 (11): 550–7. doi:10.1016/j.tips.2008.08.003. PMID 18805596.
- ↑ Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G (May 2008). "Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans". Pain. 136 (1-2): 202–10. doi:10.1016/j.pain.2008.01.024. PMID 18337008.
- ↑ Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM (November 2007). "The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans". Pain. 132 (1-2): 132–41. doi:10.1016/j.pain.2007.06.006. PMID 17659837.
- ↑ Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB (January 2007). "Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1". Proc. Natl. Acad. Sci. U.S.A. 104 (2): 660–5. doi:10.1073/pnas.0609916104. PMC 1752192
 . PMID 17194758.
. PMID 17194758. - ↑ Knotkova H, Pappagallo M, Szallasi A (2008). "Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival?". Clin J Pain. 24 (2): 142–54. doi:10.1097/AJP.0b013e318158ed9e. PMID 18209521.
- ↑ 8% Capsaicin patches. "Qutenza prescribing information" (PDF). Retrieved 23 November 2011.
- 1 2 De Petrocellis L, Schiano Moriello A, Imperatore R, Cristino L, Starowicz K, Di Marzo V (December 2012). "A re-evaluation of 9-HODE activity at TRPV1 channels in comparison with anandamide: enantioselectivity and effects at other TRP channels and in sensory neurons". British Journal of Pharmacology. 167 (8): 1643–51. doi:10.1111/j.1476-5381.2012.02122.x. PMC 3525867
 . PMID 22861649.
. PMID 22861649. - 1 2 Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM (November 2009). "Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia". Proceedings of the National Academy of Sciences of the United States of America. 106 (44): 18820–4. doi:10.1073/pnas.0905415106. PMC 2764734
 . PMID 19843694.
. PMID 19843694. - ↑ Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM (May 2010). "Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents". The Journal of Clinical Investigation. 120 (5): 1617–26. doi:10.1172/JCI41678. PMC 2860941
 . PMID 20424317.
. PMID 20424317. - ↑ Sisignano M, Angioni C, Ferreiros N, Schuh CD, Suo J, Schreiber Y, Dawes JM, Antunes-Martins A, Bennett DL, McMahon SB, Geisslinger G, Scholich K (2013). "Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice". PloS One. 8 (12): e81228. doi:10.1371/journal.pone.0081228. PMC 3857181
 . PMID 24349046.
. PMID 24349046. - ↑ Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem GD, Aich J, Kumar M, Khanna K, Singh VP, Dinda AK, Biswal S, Agrawal A, Ghosh B (2013). "Linoleic acid metabolite drives severe asthma by causing airway epithelial injury". Scientific Reports. 3: 1349. doi:10.1038/srep01349. PMC 3583002
 . PMID 23443229.
. PMID 23443229. - ↑ Wen H, Östman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A (April 2012). "20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel". The Journal of Biological Chemistry. 287 (17): 13868–76. doi:10.1074/jbc.M111.334896. PMC 3340178
 . PMID 22389490.
. PMID 22389490. - ↑ Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL (April 2012). "Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors". Proceedings of the National Academy of Sciences of the United States of America. 109 (17): 6721–6. doi:10.1073/pnas.1110460109. PMC 3340022
 . PMID 22493235.
. PMID 22493235. - ↑ Gregus AM, Dumlao DS, Wei SC, Norris PC, Catella LC, Meyerstein FG, Buczynski MW, Steinauer JJ, Fitzsimmons BL, Yaksh TL, Dennis EA (May 2013). "Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia". FASEB Journal. 27 (5): 1939–49. doi:10.1096/fj.12-217414. PMC 3633813
 . PMID 23382512.
. PMID 23382512. - ↑ Pace-Asciak CR (April 2015). "Pathophysiology of the hepoxilins". Biochimica et Biophysica Acta. 1851 (4): 383–96. doi:10.1016/j.bbalip.2014.09.007. PMID 25240838.
- ↑ Qu Q, Xuan W, Fan GH (2015). "Roles of resolvins in the resolution of acute inflammation". Cell Biology International. 39 (1): 3–22. doi:10.1002/cbin.10345. PMID 25052386.
- ↑ Serhan CN, Chiang N, Dalli J, Levy BD (2015). "Lipid mediators in the resolution of inflammation". Cold Spring Harbor Perspectives in Biology. 7 (2): a016311. doi:10.1101/cshperspect.a016311. PMID 25359497.
- ↑ Lim JY, Park CK, Hwang SW (2015). "Biological Roles of Resolvins and Related Substances in the Resolution of Pain". BioMed Research International. 2015: 830930. doi:10.1155/2015/830930. PMC 4538417
 . PMID 26339646.
. PMID 26339646. - ↑ Ji RR, Xu ZZ, Strichartz G, Serhan CN (2011). "Emerging roles of resolvins in the resolution of inflammation and pain". Trends in Neurosciences. 34 (11): 599–609. doi:10.1016/j.tins.2011.08.005. PMC 3200462
 . PMID 21963090.
. PMID 21963090. - ↑ Serhan CN, Chiang N, Dalli J (2015). "The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution". Seminars in Immunology. 27 (3): 200–15. doi:10.1016/j.smim.2015.03.004. PMC 4515371
 . PMID 25857211.
. PMID 25857211. - ↑ https://www.caymanchem.com/app/template/Product.vm/catalog/10115
- ↑ Ross RA (November 2003). "Anandamide and vanilloid TRPV1 receptors". Br. J. Pharmacol. 140 (5): 790–801. doi:10.1038/sj.bjp.0705467. PMC 1574087
 . PMID 14517174.
. PMID 14517174. - ↑ Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM (September 2005). "Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system". J. Biol. Chem. 280 (36): 31405–12. doi:10.1074/jbc.M501489200. PMID 15987694.
- ↑ Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, Laezza C, Portella G, Bifulco M, Di Marzo V (September 2006). "Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma". J. Pharmacol. Exp. Ther. 318 (3): 1375–87. doi:10.1124/jpet.106.105247. PMID 16728591.
- ↑ Starowicz K, Cristino L, Di Marzo V (2008). "TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications". Curr. Pharm. Des. 14 (1): 42–54. doi:10.2174/138161208783330790. PMID 18220817.
- 1 2 Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA (2008). "TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons". Neuron. 57 (5): 746–59. doi:10.1016/j.neuron.2007.12.027. PMC 2698707
 . PMID 18341994.
. PMID 18341994. - ↑ Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC (2010). "Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons". Neuron. 65 (5): 657–69. doi:10.1016/j.neuron.2010.02.017. PMC 2837850
 . PMID 20223201.
. PMID 20223201. - ↑ Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M (2003). "Structural determinant of TRPV1 desensitization interacts with calmodulin". Proc. Natl. Acad. Sci. U.S.A. 100 (13): 8002–6. doi:10.1073/pnas.1337252100. PMC 164702
 . PMID 12808128.
. PMID 12808128. - 1 2 Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A (2004). "Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity". J. Biol. Chem. 279 (24): 25665–72. doi:10.1074/jbc.M311515200. PMID 15066994.
- ↑ Heyman I, Rang HP (May 1985). "Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells". Neurosci. Lett. 56 (1): 69–75. doi:10.1016/0304-3940(85)90442-2. PMID 4011050.
Further reading
- Premkumar LS, Ahern GP (December 2000). "Induction of vanilloid receptor channel activity by protein kinase C". Nature. 408 (6815): 985–90. doi:10.1038/35050121. PMID 11140687.
- Immke DC, Gavva NR (October 2006). "The TRPV1 receptor and nociception". Semin. Cell Dev. Biol. 17 (5): 582–91. doi:10.1016/j.semcdb.2006.09.004. PMID 17196854.
- Heiner I, Eisfeld J, Lückhoff A (2004). "Role and regulation of TRP channels in neutrophil granulocytes". Cell Calcium. 33 (5-6): 533–40. doi:10.1016/S0143-4160(03)00058-7. PMID 12765698.
- Geppetti P, Trevisani M (2004). "Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function". Br. J. Pharmacol. 141 (8): 1313–20. doi:10.1038/sj.bjp.0705768. PMC 1574908
 . PMID 15051629.
. PMID 15051629. - Clapham DE, Julius D, Montell C, Schultz G (2005). "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels". Pharmacol. Rev. 57 (4): 427–50. doi:10.1124/pr.57.4.6. PMID 16382100.
- Szallasi A, Cruz F, Geppetti P (2006). "TRPV1: a therapeutic target for novel analgesic drugs?". Trends Mol Med. 12 (11): 545–54. doi:10.1016/j.molmed.2006.09.001. PMID 16996800.
- Pingle SC, Matta JA, Ahern GP (2007). "Capsaicin receptor: TRPV1 a promiscuous TRP channel". Handb Exp Pharmacol. 179 (179): 155–71. doi:10.1007/978-3-540-34891-7_9. PMID 17217056.
- Liddle RA (2007). "The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis". Biochim. Biophys. Acta. 1772 (8): 869–78. doi:10.1016/j.bbadis.2007.02.012. PMC 1995747
 . PMID 17428642.
. PMID 17428642.
External links
- Vanilloid receptors at the US National Library of Medicine Medical Subject Headings (MeSH)