TIM barrel

In molecular biology the TIM barrel is a conserved protein fold consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone. The structure is named after triosephosphate isomerase, a conserved metabolic enzyme. TIM barrels are one of the most common protein folds. One of the most intriguing features among members of this class of proteins is although they all exhibit the same tertiary fold there is very little sequence similarity between them. At least 15 distinct enzyme families use this framework to generate the appropriate active site geometry, always at the C-terminal end of the eight parallel beta-strands of the barrel.
Structure and composition
TIM barrels are considered α/β protein folds because they include an alternating pattern of α-helices and β-strands in a single domain. In a TIM barrel the helices and strands (usually 8 of each) form a solenoid that curves around to close on itself in a doughnut shape, topologically known as a toroid. The parallel β-strands form the inner wall of the doughnut (hence, a β-barrel), whereas the α-helices form the outer wall of the doughnut. Each β-strand connects to the next adjacent strand in the barrel through a long right-handed loop that includes one of the helices, so that the ribbon N-to-C coloring in the top view proceeds in rainbow order around the barrel. The TIM barrel can also be thought of, then, as made up of 8 overlapping, right-handed β-α-β super-secondary structures .[1]
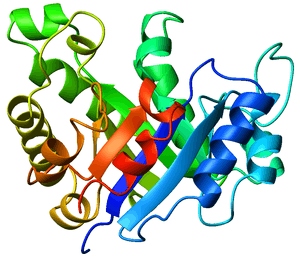
Although the ribbon diagram shows a hole in the protein's central core, the amino acid side chains are not shown in this representation. The protein's core is actually tightly packed, mostly with bulky hydrophobic amino acid residues although a few glycines are needed to allow wiggle room for the highly constrained center of the 8 approximate repeats to fit together. The packing interactions between the strands and helices are also dominated by hydrophobicity and the branched aliphatic residues valine, leucine, and isoleucine comprise about 40% of the total residues in the β-strands.[2]
Loop regions
Of the approximately 200 residues required to fully form a TIM barrel, about 160 are considered structurally equivalent between different proteins sharing this fold. The remaining residues are located on the loop regions that link the helices and strands; the loops at the C-terminal end of the strands tend to contain the active site, which is one reason this fold is so common: the residues required to maintain the structure and the residues that effect enzymatic catalysis are for the most part distinct subsets:[3] The linking loops can, in fact, be so long that they contain other protein domains. Recently, it has been demonstrated that catalytic loops can be exchanged between different TIM barrel enzymes as semiautonomous units of functional groups.[4]
See also
References
- ↑ Carl Branden and John Tooze. 1999. Introduction to Protein Structure 2nd ed. Garland Publishing: New York, NY. pp 47-50.
- ↑ Carl Branden and John Tooze. 1999. Introduction to Protein Structure 2nd ed. Garland Publishing: New York, NY. pp 47-50.
- ↑ Ochoa-Leyva, A.; et al. (2009). "Protein design through systematic catalytic loop exchange in the (beta/alpha)8 fold.". J Mol. Biol. 387 (10): 949–964. doi:10.1016/j.jmb.2009.02.022.
- ↑ Ochoa-Leyva, A.; et al. (2011). "Exploring the Structure–Function Loop Adaptability of a (β/α)8-Barrel Enzyme through Loop Swapping and Hinge Variability.". J Mol. Biol. 411 (1): 143–147. doi:10.1016/j.jmb.2011.05.027.
External links
Further reading
- R. K. Wierenga (2001). "The TIM-barrel fold: a versatile framework for efficient enzymes". FEBS Lett. 492 (3): 192–198. doi:10.1016/S0014-5793(01)02236-0. PMID 11257493.