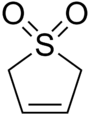
Sulfolene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,5-Dihydrothiophene 1,1-dioxide | |||
| Other names
Butadiene sulfone 3-Sulfolene | |||
| Identifiers | |||
| 77-79-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 6253 | ||
| ECHA InfoCard | 100.000.964 | ||
| PubChem | 6498 | ||
| |||
| Properties | |||
| C4H6O2S | |||
| Molar mass | 118.15 g·mol−1 | ||
| Melting point | 65 to 66 °C (149 to 151 °F; 338 to 339 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is the product of a (4+1) cycloaddition (or more technically a cheletropic reaction) between butadiene and sulfur dioxide.
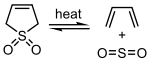
Catalytic hydrogenation yields sulfolane, a solvent used in the petrochemical industry for the extraction of aromatics from hydrocarbon streams. In the laboratory it is used as a solid source of butadiene,[2] into which it decomposes by a reverse cycloaddition.[3] However, the sulfur dioxide that is generated as a side product may cause side reactions with acid-sensitive substrates.
In electrochemical fluorination, butadiene sulfone can increase the yield of perfluorooctanesulfonyl fluoride by about 70%.[4] It is "highly soluble in anhydrous HF and increases the conductivity of the electrolyte solution."[4] In this application, it undergoes a ring opening and is fluorinated to form perfluorobutanesulfonyl fluoride.
Use as synthetic equivalent of 1,3-butadiene
Diels-Alder reaction between 1,3-butadiene and dienophiles with low reactivity usually requires prolonged heating above 100 °C. This makes a procedure rather dangerous, if neat butadiene is used, and requiring special equipment for work under elevated pressure. Alternatively, butadiene can be generated in situ by thermal sulfur dioxide extrusion from sulfolene, in which case no buildup of butadiene pressure could be expected as the liberated diene is consumed in the cycloaddition, and therefore the equilibrium of the reversible extrusion reaction acts as an internal “safety valve”.[5]

References
- ↑ Sulfolene at Sigma-Aldrich
- ↑ Sample, Thomas E.; Hatch, Lewis F (Jan 1968). "3-Sulfolene: A butadiene source for a Diels-Alder synthesis". J. Chem. Educ. 45 (1): 55. doi:10.1021/ed045p55.
- ↑ Leo Paquette (ed), Encyclopedia of Reagents for Organic Synthesis, p. 4678 ff
- 1 2 Lehmler HJ (March 2005). "Synthesis of environmentally relevant fluorinated surfactants—a review". Chemosphere. 58 (11): 1471–96. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468.
- ↑ M.A. Filatov; S. Baluschev; I.Z. Ilieva; V. Enkelmann; T. Miteva; K. Landfester; S.E. Aleshchenkov; A.V. Cheprakov (2012). "Tetraaryltetraanthra[2,3]porphyrins: Synthesis, Structure, and Optical Properties". J. Org. Chem. 77 (24): 11119–11131. doi:10.1021/jo302135q.