Sugar alcohol
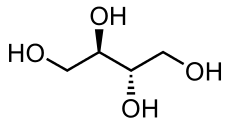
Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, that comprise a class of polyols. Contrary to what the name may suggest, a sugar alcohol is neither a sugar nor an alcoholic beverage. They are white, water-soluble solids that can occur naturally or be produced industrially from sugars. They are used widely in the food industry as thickeners and sweeteners. In commercial foodstuffs, sugar alcohols are commonly used in place of table sugar (sucrose), often in combination with high intensity artificial sweeteners to counter the low sweetness. Xylitol is perhaps the most popular sugar alcohol due to its similarity to sucrose in visual appearance and sweetness.
Production and structure
Sugar alcohols have the general formula HOCH2(CHOH)nCH2OH. In contrast, sugars have two fewer hydrogen atoms, for example HOCH2(CHOH)nCHO or HOCH2(CHOH)n−1C(O)CH2OH. The sugar alcohols differ in chain length. Most have five- or six-carbon chains, because they are derived from pentoses (five-carbon sugars) and hexoses (six-carbon sugars), respectively. They have one OH group attached to each carbon. They are further differentiated by the relative orientation (stereochemistry) of these OH groups. Unlike sugars, which tend to exist as rings, sugar alcohols do not. They can however be dehydrated to give cyclic ethers, e.g. sorbitol can be dehydrated to isosorbide.
Sugar alcohols occur naturally and at one time, mannitol was obtained from natural sources. Today, they are often obtained by hydrogenation of sugars, using Raney nickel catalysts.[1] The conversion of glucose and mannose to sorbitol and mannitol is given:
- HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO + H2 → HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHHOH
More than a million tons of sorbitol are produced in this way annually. Xylitol and lacticol are obtained similarly. Erythritol on the other hand is obtained by fermentation of glucose and sucrose.
Health effects
Sugar alcohols do not contribute to tooth decay.[2]
Consumption of sugar alcohols affects blood sugar levels, although less than of sucrose.[3][4] Sugar alcohols, with the exception of erythritol, may also cause bloating and diarrhea when consumed in excessive amounts.[5]
Common sugar alcohols
|
Both disaccharides and monosaccharides can form sugar alcohols; however, sugar alcohols derived from disaccharides (e.g. maltitol and lactitol) are not entirely hydrogenated because only one aldehyde group is available for reduction.
The simplest sugar alcohol, ethylene glycol, is sweet but notoriously toxic. The more complex sugar alcohols are for the most part nontoxic.
Sugar alcohols as food additives
| Name | Sweetness relative to sucrose | Food energy (kcal/g) |
Sweetness per food energy,
relative to sucrose |
Food energy for equal sweetness,
relative to sucrose |
|---|---|---|---|---|
| Arabitol | 0.7 | 0.2 | 14 | 7.1% |
| Erythritol | 0.8 | 0.21 | 15 | 6.7% |
| Glycerol | 0.6 | 4.3 | 0.56 | 180% |
| HSH | 0.4–0.9 | 3.0 | 0.52–1.2 | 83–190% |
| Isomalt | 0.5 | 2.0 | 1.0 | 100% |
| Lactitol | 0.4 | 2.0 | 0.8 | 125% |
| Maltitol | 0.9 | 2.1 | 1.7 | 59% |
| Mannitol | 0.5 | 1.6 | 1.2 | 83% |
| Sorbitol | 0.6 | 2.6 | 0.92 | 108% |
| Xylitol | 1.0 | 2.4 | 1.6 | 62% |
| Compare with: Sucrose |
1.0 | 4.0 | 1.0 | 100% |
As a group, sugar alcohols are not as sweet as sucrose, and they have less food energy than sucrose. Their flavor is like sucrose, and they can be used to mask the unpleasant aftertastes of some high intensity sweeteners. Sugar alcohols are not metabolized by oral bacteria, and so they do not contribute to tooth decay.[2] They do not brown or caramelize when heated.
In addition to their sweetness, some sugar alcohols can produce a noticeable cooling sensation in the mouth when highly concentrated, for instance in sugar-free hard candy or chewing gum. This happens, for example, with the crystalline phase of sorbitol, erythritol, xylitol, mannitol, lactitol and maltitol. The cooling sensation is due to the dissolution of the sugar alcohol being an endothermic (heat-absorbing) reaction, one with a strong heat of solution.[6]
Sugar alcohols are usually incompletely absorbed into the blood stream from the small intestines which generally results in a smaller change in blood glucose than "regular" sugar (sucrose). This property makes them popular sweeteners among diabetics and people on low-carbohydrate diets. However, like many other incompletely digestible substances, overconsumption of sugar alcohols can lead to bloating, diarrhea and flatulence because they are not absorbed in the small intestine. Some individuals experience such symptoms even in a single-serving quantity. With continued use, most people develop a degree of tolerance to sugar alcohols and no longer experience these symptoms. As an exception, erythritol is actually absorbed in the small intestine and excreted unchanged through urine, so it contributes no calories even though it is rather sweet.[1][5]
The table above presents the relative sweetness and food energy of the most widely used sugar alcohols. Despite the variance in food energy content of sugar alcohols, EU labeling requirements assign a blanket value of 2.4 kcal/g to all sugar alcohols.
See also
References
- 1 2 Hubert Schiweck, Albert Bär, Roland Vogel, Eugen Schwarz, Markwart Kunz, Cécile Dusautois, Alexandre Clement, Caterine Lefranc, Bernd Lüssem, Matthias Moser, Siegfried Peters "Sugar Alcohols" Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_413.pub3
- 1 2 Bradshaw, DJ; Marsh, PD (1994). "Effect of Sugar Alcohols on the Composition and Metabolism of a Mixed Culture of Oral Bacteria Grown in a Chemostat.". Caries Research. 28 (4): 251–256. doi:10.1159/000261977. PMID 8069881.
- ↑ Sue Milchovich, Barbara Dunn-Long: Diabetes Mellitus: A Practical Handbook, p. 79, 10th ed., Bull Publishing Company, 2011
- ↑ Paula Ford-Martin, Ian Blumer: The Everything Diabetes Book, p. 124, 1st ed., Everything Books, 2004
- 1 2 "Eat any sugar alcohol lately?". Yale-New Haven Hospital. 2005-03-10. Retrieved 2012-06-25.
- ↑ Cammenga, HK; LO Figura; B Zielasko (1996). "Thermal behaviour of some sugar alcohols". Journal of thermal analysis. 47 (2): 427–434. doi:10.1007/BF01983984.
External links
- "The Other 26 Sweeteners". The Sugar Association, Inc. Retrieved 2015-06-03.
- Sugar Alcohol Fact Sheet – An International Food Information Council publication