Dimercaptosuccinic acid
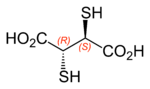 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3S)-2,3-Bis(sulfanyl)butanedioic acid | |
| Other names
(2R,3S)-2,3-Dimercaptosuccinic acid (no longer recommended) meso-2,3-Dimercaptosuccinic acid Succimer (/ˈsʌksᵻmər/) APRD01236, trade name Chemet | |
| Identifiers | |
| 304-55-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1201073 |
| ChemSpider | 2006502 |
| ECHA InfoCard | 100.005.597 |
| EC Number | 259-952-2 |
| PubChem | 2724354 |
| UNII | DX1U2629QE |
| |
| |
| Properties | |
| C4H6O4S2 | |
| Molar mass | 182.22 g/mol |
| Melting point | 125 °C (257 °F; 398 K) |
| Related compounds | |
| Related |
Tartaric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dimercaptosuccinic acid (DMSA), also called succimer, is the organosulfur compound with the formula HO2CCH(SH)CH(SH)CO2H. This colorless solid contains two carboxylic acid and two thiol groups, the latter being responsible for its mildly unpleasant odour. It occurs in two diastereomers, meso and the chiral dl forms. The meso isomer is used as a chelating agent for treatment of heavy metal toxicity and is a water-soluble and non-toxic substance.[2] When radiolabeled with technetium-99m, it helps in diagnostic testing of renal function.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system under the name succimer.[3]
Medical uses
Dimercaptosuccinic acid is indicated for the treatment of lead poisoning in children with blood level measured above 45 µg/dL. The use of DMSA is not approved for prevention of lead poisoning in anticipation of exposure in known lead contaminated environments. Its elimination half-life is 2.5-3.5 h. DMSA can cross the blood–brain barrier of mice,[4] but not that of humans, limiting its use to extracting heavy metals from parts of the body other than the central nervous system.[5][6]
DMSA facilitates urinary excretion of lead, and with sufficiently aggressive treatment, can reduce lead content in the brain.[7] DMSA also increases urinary excretion of copper and zinc.[8] DMSA improved cognitive function in rats that had been exposed to lead, but reduced cognitive function in rats that had not been exposed to lead.[7]
Another application for DMSA is for provocation of tissue heavy metals in anticipation of a urine test. This is sometimes called a "challenge" or "provoked" heavy metals test. DMSA is used to help mobilize heavy metals stored in body tissues (and therefore not typically present in the circulation) and increase the excretion of heavy metals in the urine. In a study by Howard Frumkin et al., this sort of test was shown to not reliably provide an indication of past chronic mercury exposure, something it was often used for.[9] A 2004 study by GP Archbold, et al. called the results of a DMSA challenge test "misleading" for the purposes of diagnosing mercury toxicity.[10] Moreover, DMSA is limited to extracellular distribution, which makes it unable to cross the cell membrane and chelate heavy metals from intracellular sites.
The relative activities of a series of novel monoalkyl esters of meso-2,3-dimercaptosuccinic acid (MiADMSA) have been examined as agents for the mobilization of cadmium,[11] lead [12] and arsenic [13] owing to the ability of these monoesters to cross cell membranes. The monoesters were found to be more effective than the parent compound DMSA. The complexes (monoesters of DMSA) seem to penetrate cells (not possible in the case of DMSA), which helps in targeting intracellular sites in the body and aids in the removal of toxic metal ions in the cytosol and organelles inside the cell.
Stereochemistry
The 2,3-dimercaptosuccinic acid molecule has two stereocentres (two asymmetric carbons), and can exist as three different stereoisomers. The 2S,3S and 2R,3R isomers are a pair of enantiomers, whereas the 2R,3S isomer is a meso compound and thus optically inactive.
-2%2C3-dimercaptosuccinic-acid-2D-skeletal-A-configurations-labelled.png) | 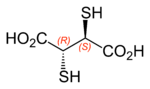 | -2%2C3-dimercaptosuccinic-acid-2D-skeletal-A-configurations-labelled.png) |
-2%2C3-dimercaptosuccinic-acid-2D-skeletal-B-configurations-labelled.png) |  | -2%2C3-dimercaptosuccinic-acid-2D-skeletal-B-configurations-labelled.png) |
(meso-2,3-dimercaptosuccinic acid) |
Preparation and reactivity
DMSA may be prepared by reacting acetylenedicarboxylic acid with sodium thiosulfate[14] or thioacetic acid followed by hydrolysis. The dimethyl ester is also known.[15]
Meso 2,3-dimercaptosuccinic acid binds to "soft" heavy metals such as Hg2+ and Pb2+, mobilizing these ions for excretion. It binds to metal cations through the thiol groups, which ionize upon complexation.
History
DMSA was first synthesized by V. Nirenburg in the Urals Polytechnic Institute, commissioned by one of the electrical enterprises of Sverdlovsk, which consumed many tons of mercury and was looking for a medicine to prevent poisoning of personnel. In 1957, it was found by Chinese scientists that DMSA can effectively treat antimony poisoning due to overdose of tartar emetic.[16] Pronounced protective effect in animal poisoning with arsenic and mercury was first shown by I. Okonishnikova in 1962. In 1984 the now-defunct Bock Pharmaceutical Company requested the FDA grant approval for Orphan drug status under the trade name Chemet and the FDA approved of this in 1991 providing exclusivity until 1998 which was conveyed to the successor Sanofi in 1996.[17][18]
See also
- Chelation therapy
- 2,3-Dimercapto-1-propanesulfonic acid
- EDTA
- Heavy metal poisoning
- Mercury poisoning
- Succinic acid
- Tartaric acid
- DMSA scan
References
- ↑ Merck Index, 11th Edition, 8854.
- ↑ Miller, Alan (June 1998). "Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity.". Alternative Medicine Review. 3 (3): 199–207. PMID 9630737.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Aasath, Jan; Dag Jacobsen; Ole Andersen; Elsa Wickstrøm (March 1995). "Treatment of Mercury and Lead Poisonings with Dimercaptosuccinic Acid (DMSA) and Sodium Dimercaptopropanesulfonate (DMPS)". Analyst. 120 (3): 853ff. doi:10.1039/an9952000853.
- ↑ Rooney, James (2007). "The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury". Toxicology. 234 (3): 145–156. doi:10.1016/j.tox.2007.02.016. PMID 17408840.
- ↑ Guzzi, GianPaolo; Caterina A.M. La Porta (2008). "Molecular mechanisms triggered by mercury". Toxicology. 244 (1): 1–12. doi:10.1016/j.tox.2007.11.002. PMID 18077077.
- 1 2 Smith D, Strupp BJ (2013). "The scientific basis for chelation: animal studies and lead chelation". Journal of Medical Toxicology. 9 (4): 326–338. doi:10.1007/s13181-013-0339-2. PMC 3846979
 . PMID 24113857.
. PMID 24113857. - ↑ Bradberry S, Sheehan T, Vale A (2009). "Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning". QJM: An International Journal of Medicine. 102 (10): 721–732. doi:10.1093/qjmed/hcp114. PMID 19700440.
- ↑ Frumkin H, Manning CC, Williams PL, et al. (February 2001). "Diagnostic chelation challenge with DMSA: a biomarker of long-term mercury exposure?". Environ. Health Perspect. 109 (2): 167–71. doi:10.1289/ehp.01109167. PMC 1240638
 . PMID 11266328.
. PMID 11266328. - ↑ Archbold GP, McGuckin RM, Campbell NA (May 2004). "Dimercaptosuccinic acid loading test for assessing mercury burden in healthy individuals". Ann. Clin. Biochem. 41 (Pt 3): 233–6. doi:10.1258/000456304323019622. PMID 15117439. Retrieved 2010-12-20.
- ↑ Jones MM, Singh PK, Gale GR, Smith AB, Atkins LM (May 1992). "Cadmium mobilization in vivo by intraperitoneal or oral administration of monoalkyl esters of meso-2,3-dimercaptosuccinic acid in the mouse". Pharmacology & Toxicology. 70 (5 Pt 1): 336–343. doi:10.1111/j.1600-0773.1992.tb00483.x. PMID 1319053.
- ↑ Walker EM Jr; Stone A; Milligan LB; Gale GR; Atkins LM; Smith AB; Jones MM; Singh PK; Basinger MA. (Nov 1992). "Mobilization of lead in mice by administration of monoalkyl esters of meso-2,3-dimercaptosuccinic acid". Toxicology. 76 (1): 79–87. doi:10.1016/0300-483x(92)90020-f. PMID 1335621.
- ↑ Kreppel H, Reichl FX, Kleine A, Szinicz L, Singh PK, Jones MM (Jul 1995). "Antidotal efficacy of newly synthesized dimercaptosuccinic acid (DMSA) monoesters in experimental arsenic poisoning in mice". Fundamental & Applied Toxicology. 26 (2): 239–245. doi:10.1093/toxsci/26.2.239. PMID 7589912.
- ↑ US 4550193, Lindemann, Martin K. O. & Lukenbach, Elvin R., "Process for the preparation of 2,3-dimercaptosuccinic acid and its lower alkyl esters", assigned to Johnson & Johnson Baby Products
- ↑ M. Gerecke; E. A. H. Friedheim; A. Brossi (1961). "Zur Kenntnis der 2,3-Dimercapto-bernsteinsäuren". Helvetica Chimica Acta. 44 (4): 955–960. doi:10.1002/hlca.19610440410.
- ↑ Liang, Y., Chu. C, Tsen, Y., Ting, K. (1957). "Studies on antibilharzial drugs. Vl. The antidotal effects of sodium dimercaptosuccinate and BAL-glucoside against tartar emetic.". Acta Physiol. Sin. 21: 24–32.
- ↑ "Search Orphan Drug Designations and Approvals". Searchable database for Orphan Designated and or Approved Products. FDA. 2013. Retrieved 5 November 2014.
- ↑ "Sanofi Buying An American Drug Concern". New York Times. New York, NY. July 17, 1996. Retrieved 5 November 2014.
Further reading
- Aposhian, H.V.; Aposhian, M.M. (1990). "Meso-2,3-dimercaptosuccinic acid: Chemical, pharmacological and toxicological properties of an orally effective metal chelating agent". Annual Review of Pharmacology and Toxicology. 30 (1): 279–306. doi:10.1146/annurev.pa.30.040190.001431. PMID 2160791.