Stratospheric sulfur aerosols
Stratospheric sulfur aerosols are sulfur-rich particles which exist in the stratosphere region of the Earth's atmosphere. The layer of the atmosphere in which they exist is known as the Junge layer, or simply the stratospheric aerosol layer. These particles consist of a mixture of sulfuric acid and water. They are created naturally, such as by photochemical decomposition of sulfur-containing gases, e.g. carbonyl sulfide. When present in high levels, e.g. after a strong volcanic eruption such as Mount Pinatubo, they produce a cooling effect, by reflecting sunlight, and by modifying clouds as they fall out of the stratosphere.[1] This cooling may persist for a few years before the particles fall out.
An aerosol is a suspension of fine solid particles or liquid droplets in a gas. The sulfate particles or sulfuric acid droplets in the atmosphere are about 0.1 to 1.0 micrometer (a millionth of a meter) in diameter.
Sulfur aerosols are common in the troposphere as a result of pollution with sulfur dioxide from burning coal, and from natural processes. Volcanos are a major source of particles in the stratosphere as the force of the volcanic eruption propels sulfur-containing gases into the stratosphere. The relative influence of volcanoes on the Junge layer varies considerably according to the number and size of eruptions in any given time period, and also of quantities of sulfur compounds released. Only stratovolcanoes containing primarily granitic rocks are responsible for these fluxes, as basaltic rock erupted in shield volcanoes doesn't result in plumes which reach the stratosphere.
Creating stratospheric sulfur aerosols deliberately is a proposed geoengineering technique which offers a possible solution to some of the problems caused by global warming. However, this will not be without side effects [2] and it has been suggested that the cure may be worse than the disease.[3]

Origins

Natural sulfur aerosols are formed in vast quantities from the SO2 ejected by volcanoes,[4] which may be injected directly into the stratosphere during very large (Volcanic Explosivity Index, VEI, of 4 or greater) eruptions. A comprehensive analysis, dealing largely with tropospheric sulfur compounds in the atmosphere, is provided by Bates et al.[5]
The IPCC AR4 says explosive volcanic events are episodic, but the stratospheric aerosols resulting from them yield substantial transitory perturbations to the radiative energy balance of the planet, with both shortwave and longwave effects sensitive to the microphysical characteristics of the aerosols.[6]
During periods lacking volcanic activity (and thus direct injection of SO2 into the stratosphere), oxidation of COS (carbonyl sulfide) dominates the production of stratospheric sulfur aerosol.[7]
Chemistry
The chemistry of stratospheric sulfur aerosols varies significantly according to their source. Volcanic emissions vary significantly in composition, and have complex chemistry due to the presence of ash particulates and a wide variety of other elements in the plume.[8]
The chemical reactions affecting both the formation and elimination of sulfur aerosols are not fully understood. It is difficult to estimate accurately, for example, whether the presence of ash and water vapour is important for aerosol formation from volcanic products, and whether high or low atmospheric concentrations of precursor chemicals (such as SO2 and H2S) are optimal for aerosol formation. This uncertainty makes it difficult to determine a viable approach for geoengineering uses of sulfur aerosol formation.
Scientific study
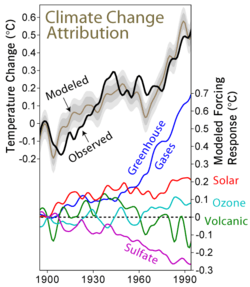
Understanding of these aerosols comes in large part from the study of volcanic eruptions,[9] notably Mount Pinatubo in the Philippines,[10] which erupted in 1991 when scientific techniques were sufficiently far advanced to study the effects carefully.[11]
The formation of the aerosols and their effects on the atmosphere can also be studied in the lab. Samples of actual particles can be recovered from the stratosphere using balloons or aircraft.[12]
Computer models can be used to understand the behaviour of aerosol particles, and are particularly useful in modelling their effect on global climate.[13]
Biological experiments in the lab, and field/ocean measurements can establish the formation mechanisms of biologically derived volatile sulfurous gases.[5]
Effects
It has been established that emission of precursor gases for sulfur aerosols is the principal mechanism by which volcanoes cause episodic global cooling.[14] The Intergovernmental Panel on Climate Change AR4 regards stratospheric sulfate aerosols as having a low level of scientific understanding. The aerosol particles form a whitish[3] haze in the sky. This creates a global dimming effect, where less of the sun's radiation is able to reach the surface of the Earth. This leads to a global cooling effect. In essence, they act as the reverse of a greenhouse gas, which tends to allow visible light from the sun through, whilst blocking infrared light emitted from the Earth's surface and its atmosphere. The particles also radiate infrared energy directly, as they lose heat into space.

All aerosols both absorb and scatter solar and terrestrial radiation. This is quantified in the Single Scattering Albedo (SSA), the ratio of scattering alone to scattering plus absorption (extinction) of radiation by a particle. The SSA tends to unity if scattering dominates, with relatively little absorption, and decreases as absorption increases, becoming zero for infinite absorption. For example, sea-salt aerosol has an SSA of 1, as a sea-salt particle only scatters, whereas soot has an SSA of 0.23, showing that it is a major atmospheric aerosol absorber.
Aerosols, natural and anthropogenic, can affect the climate by changing the way radiation is transmitted through the atmosphere. Direct observations of the effects of aerosols are quite limited so any attempt to estimate their global effect necessarily involves the use of computer models. The Intergovernmental Panel on Climate Change, IPCC, says: While the radiative forcing due to greenhouse gases may be determined to a reasonably high degree of accuracy... the uncertainties relating to aerosol radiative forcings remain large, and rely to a large extent on the estimates from global modelling studies that are difficult to verify at the present time.[15] However, they are mostly talking about tropospheric aerosol.
The aerosols have a role in the destruction of ozone[4] due to surface chemistry effects.[16] Destruction of ozone has in recent years created large holes in the ozone layer, initially over the Antarctic and then the Arctic. These holes in the ozone layer have the potential to expand to cover inhabited and vegetative regions of the planet, leading to catastrophic environmental damage.
Ozone destruction occurs principally in polar regions,[17] but the formation of ozone occurs principally in the tropics.[18] Ozone is distributed around the planet by the Brewer-Dobson circulation.[19] Therefore, the source and dispersal pattern of aerosols is critical in understanding their effect on the ozone layer.
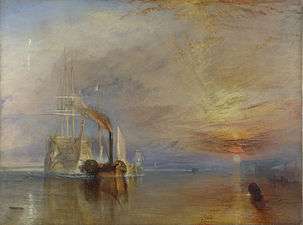
Aerosols scatter light, which affects the appearance of the sky and of sunsets. Changing the concentration of aerosols in the atmosphere can dramatically affect the appearance of sunsets. A change in sky appearance during 1816, "The Year Without A Summer" (attributed to the eruption of Mount Tambora), was the inspiration for the paintings of J. M. W. Turner. Further volcanic eruptions and geoengineering projects involving sulfur aerosols are likely to affect the appearance of sunsets significantly,[20] and to create a haze in the sky.
Aerosol particles are eventually deposited from the stratosphere onto land and ocean. Depending on the volume of particles descending, the effects may be significant to ecosystems, or may not be. Modelling of the quantities of aerosols used in likely geoengineering scenarios suggest that effects on terrestrial ecosystems from deposition is not likely to be significantly harmful.[21]
Climate engineering
The ability of stratospheric sulfur aerosols to create this global dimming effect has made them a possible candidate for use in climate engineering projects to limit the effect and impact of climate change due to rising levels of greenhouse gases.[22][23] Delivery of precursor gases such as H2S and SO2 by artillery, aircraft and balloons has been proposed.
Understanding of this proposed technique is partly based on the fact that it is the adaptation of an existing atmospheric process.[24] The technique is therefore potentially better understood than are comparable (but purely speculative) climate engineering proposals. It is also partly based on the speed of action of any such solution deployed,[25] in contrast to carbon sequestration projects such as carbon dioxide air capture which would take longer to work.[2] However, gaps in understanding of these processes exist, for example the effect on stratospheric climate and on rainfall patterns,[1] and further research is needed.[26]
See also
References
- 1 2 Bala, G.; Duffy, B.; Taylor, E. (June 2008). "Impact of geoengineering schemes on the global hydrological cycle" (Free full text). Proceedings of the National Academy of Sciences of the United States of America. 105 (22): 7664–7669. Bibcode:2008PNAS..105.7664B. doi:10.1073/pnas.0711648105. ISSN 0027-8424. PMC 2409412
 . PMID 18505844.
. PMID 18505844. - 1 2 Wigley, T.M. (2006). "A combined mitigation/geoengineering approach to climate stabilization". Science. 314 (5798): 452–4. Bibcode:2006Sci...314..452W. doi:10.1126/science.1131728. PMID 16973840.
- 1 2 Robock, A. (2008). "20 reasons why geoengineering may be a bad idea" (PDF). Bulletin of the Atomic Scientists. 64 (2): 14–18. doi:10.2968/064002006.
- 1 2 "Volcanic Sulfur Aerosols Affect Climate and the Earth's Ozone Layer". United States Geological Survey. Retrieved 17 February 2009.
- 1 2 Bates, T.S., B.K. Lamb, A. Guenther, J. Dignon and R.E. Stoiber (1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry. 14 (1–4): 315–337. doi:10.1007/BF00115242.
- ↑ Solomon, S.; et al., eds. (2007). "2.7.2.1 Radiative Effects of Volcanic Aerosols". Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007. Cambridge, United Kingdom and New York, NY, USA.: Cambridge University Press. Retrieved 2011-06-14.
- ↑ "Sulfate Aerosols".
- ↑ Mathera, T.A., C. Oppenheimer, A.G. Allen and A.J.S. McGonigle (2004). "Aerosol chemistry of emissions from three contrasting volcanoes in Italy". Atmospheric Environment. 38 (33): 5637–5649. doi:10.1016/j.atmosenv.2004.06.017.
- ↑ Baroni, M., M.H. Thiemens, R.J. Delmas, and J. Savarino (2007). "Mass-Independent Sulfur Isotopic Compositions in Stratospheric Volcanic Eruptions". Science. 315 (5808): 84–87. Bibcode:2007Sci...315...84B. doi:10.1126/science.1131754. PMID 17204647.
- ↑ Self, S., J.-X. Zhao, R.E. Holasek, R.C. Torres, and A.J. King (1997). "The Atmospheric Impact of the 1991 Mount Pinatubo Eruption". Fire and Mud: Eruptions and Lahars of Mount Pinatubo, Philippines. University of Washington Press. ISBN 0-295-97585-7.
- ↑ Jason Wolfe (5 September 2000). "Volcanoes and Climate Change". Earth Observatory. NASA. Retrieved 19 February 2009.
- ↑ Palumbo, P., A. Rotundi, V. Della Corte, A. Ciucci, L. Colangeli, F. Esposito, E. Mazzotta Epifani, V. Mennella , J.R. Brucato, F.J.M. Rietmeijer, G. J. Flynn, J.-B. Renard, J.R. Stephens, and E. Zona. "The DUSTER experiment: collection and analysis of aerosol in the high stratosphere" (PDF). Retrieved 19 February 2009.
- ↑ "Stratospheric Injections Could Help Cool Earth, Computer Model Shows". ScienceDaily. 15 September 2006. Retrieved 19 February 2009.
- ↑ Rampino MR, Self S (23 August 1984). "Sulphur-rich volcanic eruptions and stratospheric aerosols". Nature. 310 (5979): 677–9. Bibcode:1984Natur.310..677R. doi:10.1038/310677a0.
- ↑ O. Boucher; et al. (2001). "Chapter 6 Radiative Forcing of Climate Change". In J.T. Houghton; et al. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change. Retrieved 2011-06-14.
- ↑ Tie, X.; et al. (2003). "Effect of sulfate aerosol on tropospheric NOx and ozone budgets: Model simulations and TOPSE evidence". J. Geophys. Res. 108 (D4): 8364. Bibcode:2003JGRD..108.8364T. doi:10.1029/2001JD001508.
- ↑ Charles Welch. "The Ozone Hole-Ozone Destruction". Theozonehole.com. Retrieved 19 February 2009.
- ↑ Charles Welch. "Ozone". Solcomhouse.com. Retrieved 19 February 2009.
- ↑ Dylan Jones. "Brewer-Dobson Circulation" (PDF). GCC Summer School.
- ↑ Olson, D. W., R. L. Doescher, and M. S. Olson (February 2004). "When the sky ran red: The story behind The Scream". 107 (2). Sky & Telescope. pp. 29–35.
- ↑ Kravitz, B., A. Robock, L. Oman, and G. Stenchikov (2008). Acid Deposition From Stratospheric Geoengineering With Sulfate Aerosols. American Geophysical Union, Fall Meeting.
- ↑ Launder B. & J.M.T. Thompson (1996). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A. 366 (1882): 4039–56. Bibcode:2008RSPTA.366.4039C. doi:10.1098/rsta.2008.0132. PMID 18757275.
- ↑ Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?" (PDF). Climatic Change. 77 (3–4): 211–220. doi:10.1007/s10584-006-9101-y.
- ↑ Zhao, J.; Turco, R. P.; Toon, O. B. (1995). "A model simulation of Pinatubo volcanic aerosols in the stratosphere". Journal of Geophysical Research. 100: 7315. Bibcode:1995JGR...100.7315Z. doi:10.1029/94JD03325.
- ↑ Matthews, H. D.; Caldeira, K. (Jun 2007). "Transient climate–carbon simulations of planetary geoengineering" (Free full text). Proceedings of the National Academy of Sciences of the United States of America. 104 (24): 9949–9954. Bibcode:2007PNAS..104.9949M. doi:10.1073/pnas.0700419104. ISSN 0027-8424. PMC 1885819
 . PMID 17548822.
. PMID 17548822. - ↑ Andrew Charlton-Perez & Eleanor Highwood. "Costs and benefits of geo-engineering in the Stratosphere" (PDF). Retrieved 17 February 2009.