Stapled peptide
A stapled peptide is a peptide that has a synthetic brace ("staple"). Peptides with multiple, tandem staples are sometimes referred to as stitched peptides.[2][3] Peptide stapling is used to enhance pharmacologic performance of peptides.[3]
Introduction
The two primary classes of therapeutics are small molecules and protein therapeutics. It is difficult to design small molecule inhibitors of protein protein interactions[4] and protein therapeutics have poor cell penetration due to insufficient ability to diffuse across the cell membrane. Additionally, protein and peptides are often subject to proteolytic degradation. Furthermore, small peptides (such as single alpha-helices) do not exhibit significant helicity in solution due to entropic factors: this effect diminishes binding affinity.[3]
Introducing a synthetic brace (staple) helps to lock a peptide in a specific conformation reducing conformational entropy. This approach can increase target affinity, increase cell penetration, and protect against proteolytic degradation.[3][5]
Methods
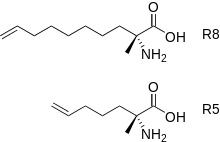
Staples synthesized using ring-closing metathesis are common.[5] This variation of olefin metathesis and its application to stapled peptides was developed by Robert H. Grubbs and Helen Blackwell in the late 1990s.[6]
Examples
In 2013, Aileron Therapeutics completed the first stapled peptide clinical trial with their growth-hormone-releasing hormone agonist ALRN-5281.[7]
References
- ↑ Douse, CH; Maas, SJ; Thomas, JC; Garnett, JA; Sun, Y; Cota, E; Tate, EW (17 October 2014). "Crystal structures of stapled and hydrogen bond surrogate peptides targeting a fully buried protein-helix interaction.". ACS Chemical Biology. 9 (10): 2204–9. doi:10.1021/cb500271c. PMID 25084543.
- ↑ Chu, Qian; Moellering, Raymond E.; Hilinski, Gerard J.; Kim, Young-Woo; Grossmann, Tom N.; Yeh, Johannes T.-H.; Verdine, Gregory L. (2015). "Towards understanding cell penetration by stapled peptides". Med. Chem. Commun. 6 (1): 111–119. doi:10.1039/c4md00131a.
- 1 2 3 4 Verdine, GL; Hilinski, GJ (2012). "Stapled peptides for intracellular drug targets.". Methods in enzymology. 503: 3–33. doi:10.1016/B978-0-12-396962-0.00001-X. PMID 22230563.
- ↑ Arkin, Michelle R.; Wells, James A. (April 2004). "Small-molecule inhibitors of protein–protein interactions: progressing towards the dream". Nature Reviews Drug Discovery. 3 (4): 301–317. doi:10.1038/nrd1343. PMID 15060526.
- 1 2 3 Walensky, LD; Bird, GH (14 August 2014). "Hydrocarbon-stapled peptides: principles, practice, and progress.". Journal of Medicinal Chemistry. 57 (15): 6275–88. doi:10.1021/jm4011675. PMID 24601557.
- ↑ Blackwell, Helen E.; Grubbs, Robert H. (17 December 1998). "Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis". Angewandte Chemie International Edition. 37 (23): 3281–3284. doi:10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V.
- ↑ "Phase 1 Safety Study of ALRN-5281 in Healthy Subjects". ClinicalTrials. U.S. National Institutes of Health. Retrieved 23 July 2015.
