Spindle neuron
| Spindle neuron | |
|---|---|
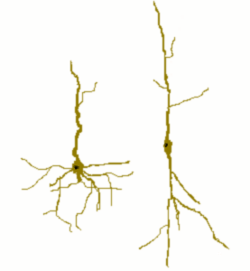 Cartoon of a spindle cell (right) compared to a normal pyramidal cell (left). | |
| Details | |
| Location | Anterior cingulate cortex and Fronto-insular cortex |
| Morphology | Unique spindle-shaped projection neuron |
| Function | Global firing rate regulation and regulation of emotional state |
| Presynaptic connections | Local input to ACC and FI |
| Postsynaptic connections | Frontal and temporal cortex. |
Spindle neurons, also called von Economo neurons (VENs), are a specific class of neurons that are characterized by a large spindle-shaped soma (or body), gradually tapering into a single apical axon in one direction, with only a single dendrite facing opposite. Other neurons tend to have many dendrites, and the polar-shaped morphology of spindle neurons is unique. A neuron's dendrites receive signals, and its axon sends them.
Spindle neurons are found in two very restricted regions in the brains of hominids—the family of species comprising humans and other great apes—the anterior cingulate cortex (ACC) and the fronto-insular cortex (FI). Recently they have been discovered in the dorsolateral prefrontal cortex of humans.[1] Spindle cells are also found in the brains of the humpback whales, fin whales, killer whales, sperm whales,[2][3] bottlenose dolphin, Risso’s dolphin, beluga whales,[4] African and Asian elephants,[5] and to a lesser extent in macaque monkeys[6] and raccoons.[7] The appearance of spindle neurons in distantly related clades suggests that they represent convergent evolution, specifically an adaptation to larger brains.
Austrian psychiatrist and neurologist Constantin von Economo (1876–1931) discovered spindle neurons and described them in 1929, which is why they are sometimes called von Economo neurons.[8]
Function of spindle neurons
Spindle neurons are relatively large cells that may allow rapid communication across the relatively large brains of great apes, elephants, and cetaceans. Although rare in comparison to other neurons, spindle neurons are abundant, and large, in humans. However, the concentration of spindle cells has been measured to be three times higher in cetaceans in comparison to humans.[3][9] They have only been found thus far in the anterior cingulate cortex (ACC), fronto-insular cortex (FI), and the dorsolateral prefrontal cortex.
Evolutionary significance
The observation that spindle neurons only occur in a highly significant group of animals (from a human point of view) has led to speculation that they are of great importance in human evolution and/or brain function. Their restriction (among the primates) to great apes leads to the hypothesis that they developed no earlier than 15-20 million years ago, prior to the divergence of orangutans from the African great apes. The discovery of spindle neurons in diverse whale species[3][4] has led to the suggestion that they are "a possible obligatory neuronal adaptation in very large brains, permitting fast information processing and transfer along highly specific projections and that evolved in relation to emerging social behaviors."[4]p. 254 Their presence in the brains of these species supports this theory, pointing towards the existence of these specialized neurons only in highly intelligent mammals, and may be an example of convergent evolution.[10] Recently, primitive forms of spindle neurons have also been discovered in macaque monkey brains[6] and raccoons.[7]
ACC spindle neurons
In 1999, Professor John Allman, a neuroscientist, and colleagues at the California Institute of Technology first published a report [11] on spindle neurons found in the anterior cingulate cortex (ACC) of hominids, but not in any other species. Neuronal volumes of ACC spindle neurons were larger in humans and the gracile (slender) chimpanzees than the spindle neurons of the robust gorillas and orangutans.
Allman and his colleagues[12] have delved beyond the level of brain infrastructure to investigate how spindle neurons function at the superstructural level, focusing on their role as 'air traffic controllers' for emotions. Allman's team proposes that spindle neurons help channel neural signals from deep within the cortex to relatively distant parts of the brain.
Specifically, Allman's team[13] found signals from the ACC are received in Brodmann's area 10, in the frontal polar cortex, where regulation of cognitive dissonance (disambiguation between alternatives) is thought to occur. According to Allman, this neural relay appears to convey motivation to act, and concerns the recognition of error. Self-control – and avoidance of error – is thus facilitated by the executive gatekeeping function of the ACC, as it regulates the interference patterns of neural signals between these two brain regions.
In humans, intense emotion activates the anterior cingulate cortex, as it relays neural signals transmitted from the amygdala (a primary processing center for emotions) to the frontal cortex, perhaps by functioning as a sort of lens to focus the complex texture of neural signal interference patterns. The ACC is also active during demanding tasks requiring judgment and discrimination, and when errors are detected by an individual. During difficult tasks, or when experiencing intense love, anger, or lust, activation of the ACC increases. In brain imaging studies, the ACC has specifically been found to be active when mothers hear infants cry, underscoring its role in affording a heightened degree of social sensitivity.
The ACC is a relatively ancient cortical region, and is involved with many autonomic functions, including motor and digestive functions, while also playing a role in the regulation of blood pressure and heart rate. Significant olfactory and gustatory capabilities of the ACC and fronto-insular cortex appear to have been usurped, during recent evolution, to serve enhanced roles related to higher cognition – ranging from planning and self-awareness to role playing and deception. The diminished olfactory function of humans, compared to other primates, may be related to the fact that spindle cells located at crucial neural network hubs have only two dendrites rather than many, resulting in reduced neurological integration.
Fronto-insular spindle neurons
At a Society for Neuroscience meeting in 2003, Allman reported on spindle cells his team found in another brain region, the fronto-insular cortex, a region which appears to have undergone significant evolutionary adaptations in mankind – perhaps as recently as 100,000 years ago.
This fronto-insular cortex is closely connected to the insula, a region that is roughly the size of a thumb in each hemisphere of the human brain. The insula and fronto-insular cortex are part of the insular cortex, wherein the elaborate circuitry associated with spatial awareness are found, and where self-awareness and the complexities of emotion are thought to be generated and experienced. Moreover, this region of the right hemisphere is crucial to navigation and perception of three-dimensional rotations.
Spindle neuron concentrations
ACC
The largest number of ACC spindle neurons are found in humans, fewer in the gracile great apes, and fewest in the robust great apes. In both humans and bonobos they are often found in clusters of 3 to 6 neurons. They are found in humans, bonobos, common chimpanzees, gorillas, orangutans, some cetaceans, and elephants.[14]:245 While total quantities of ACC spindle neurons were not reported by Allman in his seminal research report (as they were in a later report describing their presence in the frontoinsular cortex, below), his team's initial analysis of the ACC layer V in hominids revealed an average of ~9 spindle neurons per section for orangutans (rare, 0.6% of section cells), ~22 for gorillas (frequent, 2.3%), ~37 for chimpanzees (abundant, 3.8%), ~68 for bonobos (abundant/clusters, 4.8%), ~89 for humans (abundant/clusters, 5.6%).[15]
Fronto-insula
All of the primates examined had more spindle cells in the fronto-insula of the right hemisphere than in the left. In contrast to the higher number of spindle cells found in the ACC of the gracile bonobos and chimpanzees, the number of fronto-insular spindle cells was far higher in the cortex of robust gorillas (no data for Orangutans was given). An adult human had 82,855 such cells, a gorilla had 16,710, a bonobo had 2,159, and a chimpanzee had a mere 1,808 – despite the fact that chimpanzees and bonobos are great apes most closely related to humans.
Dorsolateral PFC
Von Economo neurons have been located in the Dorsolateral prefrontal cortex of humans[1] and elephants.[5] In humans they have been observed in higher concentration in Brodmann area 9 (BA9) – mostly isolated or in clusters of 2, while in Brodmann area 24 (BA24) they have been found mostly in clusters of 2-4.[1]
Related pathologies
Abnormal spindle neuron development may be linked to several psychotic disorders, typically those characterized by distortions of reality, disturbances of thought, disturbances of language, and withdrawal from social contact. Altered spindle neuron states have been implicated in both schizophrenia and autism, but research into these correlations remains at a very early stage. Frontotemporal dementia involves loss of mostly spindle neurons.[16] An initial study suggested that Alzheimer's disease specifically targeted Von Economo neurons; this study was performed with end-stage Alzheimer brains in which cell destruction was widespread, but later, it was found that Alzheimer's disease doesn't affect the spindle neurons.
References
- 1 2 3 Fajardo; Escobar, M.I.; Buriticá, E.; Arteaga, G.; Umbarila, J.; Casanova, M.F.; Pimienta, H.; et al. (4 March 2008). "Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans.". Neuroscience Letters. 435 (3): 215–218. doi:10.1016/j.neulet.2008.02.048. PMID 18355958.
- ↑ Coghlan, A. (27 November 2006). "Whales boast the brain cells that 'make us human'". New Scientist.
- 1 2 3 Hof, P. R., Van der Gucht, E. (Jan 2007). "Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae)". Anat Rec. 290 (1): 1–31. doi:10.1002/ar.20407. PMID 17441195.
- 1 2 3 Butti C, Sherwood CC, Hakeem AY, Allman JM, Hof PR (July 2009). "Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans.". The Journal of Comparative Neurology. 515 (2): 243–59. doi:10.1002/cne.22055. PMID 19412956.
- 1 2 Hakeem, A. Y.; Sherwood, C. C.; Bonar, C. J.; Butti, C.; Hof, P. R.; Allman, J. M. (2009). "Von Economo neurons in the elephant brain". The Anatomical Record (Hoboken). 292 (2): 242–8. doi:10.1002/ar.20829. PMID 19089889.
- 1 2 http://www.sciencedaily.com/releases/2012/05/120521115353.htm
- 1 2 Lambert KG, Bardi M, Landis T, Hyer MM, Rzucidlo A, Gehrt S, Anchor C, Jardim Messeder D, Herculano-Houzel S (2014). "Behind the Mask: Neurobiological indicants of emotional resilience and cognitive function in wild raccoons (Procyon lotor)". Society for Neuroscienc.
- ↑ von Economo, C., & Koskinas, G. N. (1929). The cytoarchitectonics of the human cerebral cortex. London: Oxford University Press
- ↑ Coghlan, A. (27 November 2006). "Whales boast the brain cells that 'make us human'". New Scientist.
- ↑ Hakeem, Atiya Y.; Sherwood, Chet C.; Bonar, Christopher J.; Butti, Camilla; Hof, Patrick R.; Allman, John M. (16 December 2009). "Von Economo Neurons in the Elephant Brain". The Anatomical Record. 292 (2): 242–248. doi:10.1002/ar.20829. PMID 19089889.
- ↑ Nimchinsky, EA; Gilissen, E; Allman, JM; Perl, DP; Erwin, JM; Hof, PR (Apr 1999). "A neuronalmorphologic type unique to humans and great apes". Proc Natl Acad Sci U S A. 96 (9): 5268–73. PMID 10220455.
- ↑ Allman, JM; Hakeem, A; Erwin, JM; Nimchinsky, E; Hof, P (May 2001). "The anterior cingulatecortex. The evolution of an interface between emotion and cognition". Ann N Y Acad Sci. 935: 107–17. doi:10.1111/j.1749-6632.2001.tb03476.x. PMID 11411161.
- ↑ Allman, J; Hakeem, A; Watson, K (Aug 2002). "Two phylogenetic specializations in the humanbrain". Neuroscientist. 8 (4): 335–46. doi:10.1177/107385840200800409. PMID 12194502.
- ↑ Hakeem, Atiya Y.; Chet. C. Sherwood; Christopher J. Bonar; Camilla Butti; Patrick R. Hof; John M. Allman (December 2009). "Von Economo Neurons in the Elephant Brain". The Anatomical Record. 292 (2): 242–248. doi:10.1002/ar.20829. PMID 19089889.
- ↑ Allman J, Hakeem A, Watson K (Aug 2002). "Two phylogenetic specializations in the human brain". Neuroscientist. 8 (4): 335–46. doi:10.1177/107385840200800409. PMID 12194502.
- ↑ Seeley, W. W., Carlin, D. A., Allman, J. M., Macedo, M. N., Bush, C., Miller, B. L., & DeArmond, S. J. (Dec 2006). "Early fronto-temporal dementia targets neurons unique to apes and humans". Ann. Neurol. 60 (6): 660–7. doi:10.1002/ana.21055. PMID 17187353.
- General References
- Allman J, Hakeem A, Watson K (Aug 2002). "Two phylogenetic specializations in the human brain". Neuroscientist. 8 (4): 335–46. doi:10.1177/107385840200800409. PMID 12194502.
External links
- TaipeiTimes.com – Know Thyself and Others
- "Well-wired whales" Michael Balter (2006) ScienceNOW Daily News. 27 November
- "Brain Cells for Socializing" Smithsonian, June 2009