Sodium ferrioxalate
 | |
| Names | |
|---|---|
| IUPAC name
Sodium iron(III) oxalate | |
| Other names
Sodium ferrioxalate Sodium ferric oxalate | |
| Identifiers | |
| 5936-14-1 | |
| Properties | |
| Na3[Fe(C2O4)3]
Na3[Fe(C2O4)3].xH2O | |
| Molar mass | 388.88 g/mol - anhydrous sodium trioxalatoferrate (III) 388.88 + x(18.01) g/mol - hydrated sodium trioxalatoferrate (III) |
| Appearance | lime green hydrated crystals |
| Density | 1.97 g/cm3 at 17 °C |
| 32.5pts per 100pts solvent, cold water, 182pts per 100pts, boiling water[1] | |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | Corrosive. Eye, respiratory and skin irritant. |
| R-phrases | R20 R21 R22 R34 R36 R37 R38 |
| Related compounds | |
| Other anions |
Potassium ferrioxalate |
| Related compounds |
Iron(II) oxalate Iron(III) oxalate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
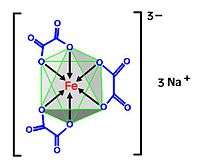
Sodium ferrioxalate, also known as sodium oxalatoferrate, is a chemical compound with the formula Na3[Fe(C2O4)3], where iron is in the +3 oxidation state. It is an octahedral transition metal complex in which three bidentate oxalate ions act as ligands bound to an iron centre. Sodium acts as a counterion, balancing the -3 charge of the complex. Crystals of the hydrated form of the complex, Na3[Fe(C2O4)3].xH2O, are lime green in colour. In solution the complex dissociates to give the ferrioxalate anion, [Fe(C2O4)3]3−, which appears a deep apple green in colour.
Bonding
The bonds to the iron atom are dative covalent bonds where the ligands, (oxalate ions, blue), donate a lone pair into the empty p and d orbitals of the transition metal (iron, red), atom. The three oxalate ions donate 12 electrons in all and Fe-III has three electrons in the d orbitals leaving 13 empty places in the remaining d and p orbitals.
Solubility
This compound is very soluble in hot water, (182 parts per 100 parts solvent by mass), but a lot less soluble in cold water, (32 parts per 100 parts solvent), about the solubility of sodium chloride. It is not appreciably soluble in ethanol or ethanol water mixtures which are more than 50% ethanol by mass. It is somewhat more soluble in water than the corresponding potassium salt.
Preparation
The crystals pictured were synthesised by mixing solutions of sodium oxalate and ferric oxalate and waiting a few hours for the brown colour of the ferric oxalate to be replaced with the green colour of the complex anion. This complex is relatively inert and the equilibrium is attained only slowly at room temperature. The ferric oxalate was made by dissolving rust in oxalic acid and filtering off any residual insolubles. The solution was evaporated at just below boiling until small crystals appeared on the bottom indicating the solution was then hot and saturated. The solution was allowed to cool in a beaker sitting on a large aluminium block. The thermal mass of the block allowed sufficiently slow cooling over night to produce crystals a few millimetres long. These larger crystals are pictured at the upper left.
Fe2(C2O4)3 + 3 Na2(C2O4) → 2 Na3[Fe(C2O4)3]
Stoichiometry was not worried about and an excess of sodium oxalate was added, this is a lot less soluble in hot water than the ferrioxalate and crystallizes out first. The intensity of the green colour was used as a guide to concentration of the solution with respect to the complex. A few drops of 100 vol hydrogen peroxide were periodically added during the evaporation to maintain the iron in the III oxidation state and any insoluble ferrous oxalate was removed if it precipitated out.
The smaller crystals were recovered from the solution by placing it in the freezer after the large crystals had been removed. The smallest crystals, pictured at the lower right were precipitated from the cold solution by addition of methylated spirit.
Isomerism
The ferrioxalate complex demonstrates optical activity since there are two non-superimposable stereoisomers of the complex. This is described in more detail under potassium ferrioxalate. Theoretically the two stereoisomers could be separated by crystallization of a diastereomeric salt of the optically inactive racemic mixture of ferrioxalate ions with an optically active cation, such as methylethylpropylammonium ion which is one pure enantomer. Thus methylethylpropylammonium ferrioxalate should crystallize out to produce crystals which are non superimposable mirror images. These would be Λ-methylethylpropylammonium Λ-ferrioxalate and Λ-methylethylpropylammonium Δ-ferrioxalate.
Photoreduction
In solution the ferrioxalate complex is decomposed by light. This is described in more detail under potassium ferrioxalate. Some samples of the crystals were exposed to direct sunlight for a few hours, the larger crystals did not appear to be affected, however solutions and small crystals so exposed did change colour to a different shade of green.
If a solution containing both green ferrioxalate ions and colourless free oxalate ions is exposed to strong light, such as direct sunlight, the light allows the Iron-III to oxidize one of the oxalate ligands to carbon dioxide and gives the orange-brown ferrooxalate complex ion which is coordinated around an Iron-II centre, however, when placed in the dark the Iron-II is re-oxidized to Iron-III by the oxygen in the atmosphere and the green ferrioxalate complex ion re-forms. The orange-brown Iron-II complex starts to appear after around ten minutes exposure and after the passage of a few hours in direct sunlight more than half of the green Iron-III complex had been reduced. The re-oxidation in the dark is equally slow and observable under ambient electric lighting. If this process is allowed to repeat over many months, such as leaving a container outside where it is exposed to the sun each day, eventually almost all of the oxalate ions present are oxidized to carbonate and the iron remains as Ferric Hydroxide, Fe(OH)3.
This indicates that when exposed to the environment, particularly if that environment is damp the ferrioxalate ion is quite unstable and gradually decomposes via the above redox processes into much more stable and common compounds.
This light catalyzed redox reaction once formed the basis of some photographic processes, however due to their insensitivity and the ready availability of digital photography these processes have become obsolete and all but forgotten.
Uses
In contemporary times ferrioxalate salts, usually the potassium salt, are used as examples of a transition metal ligand complexes which can be easily synthesized by high school, college or undergraduate university students to introduce them to transition metal ligand chemistry, as well as to redox chemistry in now-obsolete photographic processes.
See also
A number of other iron oxalates are known
References
- ↑ C.R.C. Handbook of Chemistry and Physics, 62nd Ed., page B149, ISBN 0-8493-0462-8, 1981, CRC Press