SmURFP
.gif)
Small ultra red fluorescent protein (smURFP) is a new class of far-red fluorescent protein evolved from a cyanobacterial (Trichodesmium erythraeum) phycobiliprotein, α-allophycocyanin.[1] Native α-allophycocyanin requires an exogenous protein, known as a lyase, to attach the chromophore, phycocyanobilin. Phycocyanobilin is not present in mammalian cells. smURFP was evolved to covalently attach phycocyanobilin without a lyase and fluoresce, covalently attach biliverdin (ubiquitous to mammalian cells) and fluoresce, blue-shift fluorescence to match the organic fluorophore, Cy5, and not inhibit E. coli growth. smURFP was found after 12 rounds of random mutagenesis and manually screening 10,000,000 bacterial colonies.
Properties
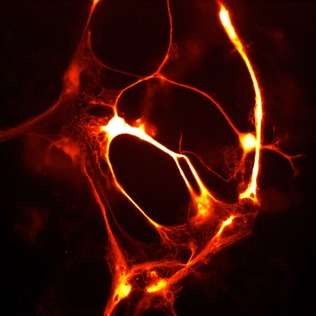
smURFP is a homodimer with absorption and emission maximum of 642 nm and 670 nm, respectively. A tandem dimer smURFP (TDsmURFP) was created and has similar properties to smURFP. smURFP is extremely stable with a protein degradation half-life of 17 hour and 33 hour without and with chromophore (biliverdin), respectively. This is comparable to the jellyfish-derived enhanced green fluorescent protein (eGFP) protein degradation half-life of 24 hour.[2] smURFP is extremely photostable and out performs mCherry[3] and tdTomato[3] in living cells. The extinction coefficient (180,000 M−1 cm−1) of smURFP is extremely large and has a modest quantum yield (0.20), which makes it comparable biophysical brightness to eGFP and ~2-fold brighter than most red or far-red fluorescent proteins derived from coral. Despite being a homodimer, all tested N- and C- terminal fusions show correct cellular localization, including the difficult fusion to α-tubulin and Lamin B1 (Figure).[1]
An Excel sheet of the smURFP absorbance, excitation, and emission spectra can be downloaded here.

Chromophore (Biliverdin) Availability in Cells and Mice

Despite showing comparable biophysical brightness to eGFP when purified protein was normalized, this was not seen in living cells. This suggested there was not enough chromophore (biliverdin) within cells. Addition of biliverdin increased fluorescence, but smURFP with biliverdin was not comparable to eGFP. Biliverdin has two carboxylates at neutral pH and this is inhibiting cellular entry. Biliverdin dimethyl ester is a more hydrophobic analog and readily crosses the cellular membrane. smURFP with biliverdin dimethyl ester shows comparable fluorescence to eGFP in cells and is brighter than bacterial phytochrome fluorescent proteins.
In mice, smURFP fluorescence is visible in HT1080 tumor xenografts without exogenous biliverdin, but fluorescence is less than coral-derived red fluorescent proteins, mCherry[3] and mCardinal.[4] Visible fluorescence is not always usable fluorescence and fluorescent proteins should always be compared to other useful, genetically encoded fluorescent proteins. Intravenous injection of exogenous biliverdin or biliverdin dimethyl ester does not increase fluorescence of smURFP expressed in tumors after 1 to 24 hours. Mass spectrometry showed that the ester groups were rapidly removed from biliverdin dimethyl ester. Addition of 25 μM biliverdin or biliverdin dimethyl ester dramatically increased fluorescence of excised tumors and smURFP is present without chromophore. Further research is necessary to optimize chromophore availability in mice to obtain fluorescence comparable or greater than coral-derived red fluorescent proteins.
Adding Chromophore to Cells
Biliverdin dimethyl ester, biliverdin, and phycocyanobilin are commercially available from Frontier Scientific. Biliverdin dimethyl ester, biliverdin, or phycocyanobilin is dissolved in DMSO at a concentration of 5 mM. The solution is very dark and pipette vigorously to ensure all is dissolved. Biliverdin dimethyl ester is not soluble in common buffers, including phosphate buffered saline (PBS) or Hank's balanced salt solution (HBSS). Add 1-5 μM biliverdin dimethyl ester in media or buffer containing 10% fetal bovine serum (FBS). Add 25 μM biliverdin (not as membrane permeant) to cells. Incubate smURFP with chromophore for as long as possible to increase protein accumulation caused by enhanced protein stability with chromophore. Leave chromophore for a minimum of 3 hours. Remove chromophore, wash with media containing 10% FBS, and image in media (lacking phenol red) or imaging buffer.
Fluorescence Imaging of the Cell Cycle
Pioneering work by Atsushi Miyawaki and coworkers developed the fluorescent ubiquitination-based cell cycle indicator (FUCCI), which enables fluorescence imaging of the cell cycle. Originally, a green fluorescent protein, mAG, was fused to hGem(1/110) and an orange fluorescent protein (mKO2) was fused to hCdt1(30/120). Note, these fusions are fragments that contain a nuclear localization signal and ubiquitination sites for degradation, but are not functional proteins. The green fluorescent protein is made during the S, G2, or M phase and degraded during the G0 or G1 phase, while the orange fluorescent protein is made during the G0 or G1 phase and destroyed during the S, G2, or M phase.[5] A far-red and near-infrared FUCCI was developed using a cyanobacteria-derived fluorescent protein (smURFP) and a bacteriophytochrome-derived fluorescent protein (movie found at this link).[1]
Sequences and Obtaining Plasmids
GenBank/EMBL/DDBJ Accession Codes can be found at the following links: smURFP KX449134 & TDsmURFP, KX449135.
DNA plasmids can be obtained from Addgene at the following link: Erik Rodriguez Lab Plasmids include bacterial and mammalian expression and lentiviral transfer vectors.
References
- 1 2 3 Rodriguez, Erik A.; Tran, Geraldine N.; Gross, Larry A.; Crisp, Jessica L.; Shu, Xiaokun; Lin, John Y.; Tsien, Roger Y. (2016-08-01). "A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein". Nature Methods. doi:10.1038/nmeth.3935. ISSN 1548-7105. PMID 27479328.
- ↑ Stack, J. H.; Whitney, M.; Rodems, S. M.; Pollok, B. A. (2000-12-01). "A ubiquitin-based tagging system for controlled modulation of protein stability". Nature Biotechnology. 18 (12): 1298–1302. doi:10.1038/82422. ISSN 1087-0156. PMID 11101811.
- 1 2 3 Shaner, Nathan C.; Campbell, Robert E.; Steinbach, Paul A.; Giepmans, Ben N. G.; Palmer, Amy E.; Tsien, Roger Y. (2004-12-01). "Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein". Nature Biotechnology. 22 (12): 1567–1572. doi:10.1038/nbt1037. ISSN 1087-0156. PMID 15558047.
- ↑ Chu, Jun; Haynes, Russell D; Corbel, Stéphane Y; Li, Pengpeng; González-González, Emilio; Burg, John S; Ataie, Niloufar J; Lam, Amy J; Cranfill, Paula J. "Non-invasive intravital imaging of cellular differentiation with a bright red-excitable fluorescent protein". Nature Methods. 11 (5): 572–578. doi:10.1038/nmeth.2888. PMC 4008650
 . PMID 24633408.
. PMID 24633408. - ↑ Sakaue-Sawano, Asako; Kurokawa, Hiroshi; Morimura, Toshifumi; Hanyu, Aki; Hama, Hiroshi; Osawa, Hatsuki; Kashiwagi, Saori; Fukami, Kiyoko; Miyata, Takaki (2008-02-08). "Visualizing spatiotemporal dynamics of multicellular cell-cycle progression". Cell. 132 (3): 487–498. doi:10.1016/j.cell.2007.12.033. ISSN 1097-4172. PMID 18267078.