Slow-wave sleep
Slow-wave sleep (SWS), often referred to as deep sleep, consists of stage three of non-rapid eye movement sleep, according to the Rechtschaffen & Kales (R & K) standard of 1968.[2] There is not a clear distinction between stages three and four. Stage three has 20-50 percent delta activity, whereas stage four has more than 50 percent.[3] As of 2008, the American Academy of Sleep Medicine (AASM) has discontinued the use of stage four,[4][5][6] such that the previous stages three and four now are combined as stage three. An epoch (30 seconds of sleep) which consists of 20% or more slow-wave (delta) sleep is now considered to be stage three.
This period of sleep is called slow-wave sleep because the EEG activity is synchronized, producing slow waves with a frequency of less than 1 Hz and a relatively high amplitude. The first section of the wave signifies a down state, which is an inhibition period in which the neurons in the neocortex are silent. This is the period when the neocortical neurons are able to rest. The second section of the wave signifies an up state, which is an excitation period in which the neurons fire briefly at a high rate. The former state is a hyperpolarizing phase and the latter is a depolarizing phase. The principal characteristics during slow-wave sleep that contrast with REM sleep are moderate muscle tone, slow or absent eye movement, and lack of genital activity.[7]
Slow-wave sleep is considered important to consolidate new memories.[8] This is sometimes referred to as "sleep-dependent memory processing". Impaired memory consolidation has been effected in individuals with primary insomnia who thus do not perform as well as normal patients in memory tasks following a period of sleep. Furthermore, slow-wave sleep improves declarative memory (which includes semantic and episodic memory). A central model has been hypothesized that the long-term memory storage is facilitated by an interaction between the hippocampal and neocortical networks.[9] In several studies, after the subjects have had training to learn a declarative memory task, the density of human sleep spindles was significantly higher compared to the non-learning control task. This is the result of the spontaneously occurring wave oscillations that account for the intracellular recordings from thalamic and cortical neurons.[10]
Sleep deprivation studies with humans suggest that the primary function of slow-wave sleep may be to allow the brain to recover from its daily activities. Glucose metabolism in the brain increases as a result of tasks that demand mental activity.[11] Another function affected by slow-wave sleep is the secretion of growth hormone, which is always greatest during this stage.[12] It is also thought to be responsible for a decrease in sympathetic and increase in parasympathetic neural activity.[12]
Discussion
The highest arousal thresholds (i.e. difficulty of awakening, such as by a sound of a particular volume) are observed in deep sleep. A person will typically feel more groggy when awoken from slow-wave sleep, and indeed, cognitive tests administered after awakening then indicate that mental performance is somewhat impaired for periods of up to 30 minutes, relative to awakenings from other stages. This phenomenon has been called "sleep inertia".
After sleep deprivation there is a sharp rebound of SWS, that is, the following bout of sleep will include more and deeper SWS than normal. The duration of slow-wave sleep is determined by the previous duration of this stage as well as the duration of prior wakefulness.[12]
The major factor determining how much slow-wave sleep is observed in a given sleep period is the duration of preceding wakefulness, likely related to accumulation of sleep-promoting substances in the brain. Some of the factors known to increase slow-wave sleep in the sleep period that follows them include body heating (as by immersion in a hot tub or sauna) and intense prolonged exercise. Studies have shown that slow-wave sleep is enabled when brain temperature surpasses a certain threshold. It is hypothesized that the threshold is regulated by circadian and homeostatic processes.[13] In healthy sleepers, a very low carbohydrate diet over the short-term promotes increases in the percentage of SWS (deep sleep stage four, now often called stage three) and a reduction in the percentage of REM sleep (dreaming sleep) compared to the control with a mixed diet - the sleep changes may be linked to the metabolism of the fat content of the very low carbohydrate diet.[14]
In addition to these factors, the duration of SWS periods can be increased by the ingestion of certain SSRI, and other antidepressants, whereas the effects of THC on SWS remain controversial.[15][16] In instances such as these, total sleep time (TST) is often unaffected due to circadian rhythms, a person's alarm clock, or early morning obligations. This increase of SWS can lead to increased REM latency and a decrease in REM duration. If the total time spent in REM sleep is decreased long enough and repeatedly over a substantial number of nights a "REM rebound" will occur in response to the removal of its inhibitor. An increase in REM sleep is believed to produce symptoms of depression and bipolar disorder in many patients for an amount of time relative to the severity of the previous REM suppression. It is debatable whether this explains the return in symptoms of depression disorder after removal of SSRI medications.
The chemical gamma-hydroxybutyric acid (GHB) is known to promote SWS.[17] In the United States, the Food and Drug Administration permits the use of GHB under the trade name Xyrem to reduce cataplexy attacks and excessive daytime sleepiness in patients with narcolepsy.
Reduced slow-wave sleep (SWS) may predict high blood pressure in older men.[18]
A study from the Department of Endocrinology at Boston Children's Hospital, an affiliate of Harvard Medical School, indicated that regular deep sleep in children is helpful in triggering the steady release of the hormones that cause puberty.[19]
Neural control of slow-wave sleep
Several neurotransmitters are involved in sleep and waking patterns: acetylcholine, norepinephrine, serotonin, histamine, and orexin.[20] Neocortical neurons fire spontaneously during slow-wave sleep, thus they seem to play a role during this period of sleep. Also, these neurons appear to have some sort of internal dialogue, which accounts for the mental activity during this state where there is no information from external signals because of the synaptic inhibition at the thalamic level. The rate of recall of dreams during this state of sleep is relatively high compared to the other levels. This indicates that the mental activity is closer to real life events.[10]
Functions of slow-wave sleep
Slow-wave sleep is necessary for survival. Some animals, such as dolphins and birds, have the ability to sleep with only one hemisphere of the brain, leaving the other hemisphere awake to carry out normal functions and to remain alert.
Physical healing and growth
Slow-wave sleep is the constructive phase of sleep for recuperation of the mind-body system in which it rebuilds itself after each day. Substances that have been ingested into the body while an organism is awake are synthesized into complex proteins of living tissue. Growth hormones are also secreted to facilitate the healing of muscles as well as repairing damage to any tissues. Lastly, glial cells within the brain are restored with sugars to provide energy for the brain.[21]
Effects of sleep deprivation
J. A. Horne (1978) reviewed several experiments with humans and concluded that sleep deprivation has no effects on people’s physiological stress response or ability to perform physical exercise. It did, however, have an effect on cognitive functions. Some people reported distorted perceptions or hallucinations and lack of concentration on mental tasks. Thus, the major role of sleep does not appear to be rest for the body, but rest for the brain.
When sleep-deprived humans sleep normally again, the recovery percentage for each stage of sleep is not the same. Only seven percent of stages one and two are regained, but 68 percent of stage-four slow-wave sleep and 53 percent of REM sleep are regained. This suggests that stage-four sleep (known today as the deepest part of stage-three sleep) is more important than the other stages.
During slow-wave sleep, there is a significant decline in cerebral metabolic rate and cerebral blood flow. The activity falls to about 75 percent of the normal wakefulness level. The regions of the brain that are most active when awake have the highest level of delta waves during slow-wave sleep. This indicates rest is geographical. The “shutting down” of the brain accounts for the grogginess and confusion if someone is awakened during deep sleep since it takes the cerebral cortex time to resume its normal functions.
According to J. Siegel (2005), sleep deprivation results in the build-up of free radicals and superoxides in the brain. Free radicals are oxidizing agents that have one unpaired electron, making them highly reactive. These free radicals interact with electrons of biomolecules and damage cells. In slow-wave sleep, the decreased rate of metabolism reduces the creation of oxygen byproducts, thereby allowing the existing radical species to clear. This is a means of preventing damage to the brain.[22]
Problems associated with slow-wave sleep
Bedwetting, night terrors, and sleep-walking are all common behaviors that can occur during stage three of sleep. These occur most frequently amongst children, who then generally outgrow them.[11] Another problem that may arise is sleep-related eating disorder. An individual will sleep-walk leaving his or her bed in the middle of the night seeking out food, and will eat not having any memory of the event in the morning.[11] Over half of individuals with this disorder become overweight.[23] Sleep-related eating disorder can usually be treated with dopaminergic agonists, or topiramate, which is an anti-seizure medication. This nocturnal eating throughout a family suggests that heredity may be a potential cause of this disorder.[11]
Individual differences in slow-wave sleep
Though SWS is fairly consistent within the individual, it can vary across individuals. Age and gender have been noted as two of the biggest factors that affect this period of sleep. Aging is inversely proportional to the amount of SWS beginning by midlife and therefore, SWS declines with age. Sex differences have also been found, such that females tend to have higher levels of SWS compared to males, at least up until menopause. There have also been studies that have shown differences between races. The results showed that there was a lower percentage of SWS in African Americans compared to Caucasians, but since there are many influencing factors (e.g. body mass index sleep-disordered breathing, obesity, diabetes, and hypertension) this potential difference must be investigated further.[24]
Electroencephalographic characteristics
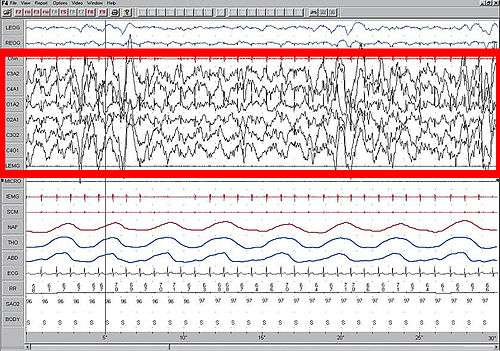
High amplitude EEG is highlighted in red.
Large 75-microvolt (0.5–3 Hz) delta waves predominate the electroencephalogram (EEG). Stage N3 is defined by the presence of 20% delta waves in any given 30-second epoch of the EEG during sleep, by the current 2007 AASM guidelines.[6]
Longer periods of SWS occur in the first part of the night, primarily in the first two sleep cycles (roughly three hours). Children and young adults will have more total SWS in a night than older adults. The elderly may not go into SWS at all during many nights of sleep.
Slow-wave sleep is an active phenomenon probably brought about by the activation of serotonergic neurons of the raphe system.
The slow wave seen in the cortical EEG is generated through thalamocortical communication through the thalamocortical neurons.[25] In the TC neurons this is generated by the "slow oscillation" and is dependent on membrane potential bistability, a property of these neurons due to an electrophysiological component known as "I t window". "I t window" is due to the overlap underneath activation and inactivation curves if plotted for T-type calcium channels (inward current). If these two curves are multiplied, and another line superimposed on the graph to show a small Ik leak current (outward), then the interplay between these inward (I t window) and outward (small Ik leak), three equilibrium points are seen at −90, −70 and −60 mv, −90 and −60 being stable and −70 unstable. This property allows the generation of slow waves due to an oscillation between two stable points. It is important to note that in in vitro, mGluR must be activated on these neurons to allow a small Ik leak, as seen in in vivo situations.
See also
References
Notes
- ↑ Lesku, J. A.; Meyer, L. C. R.; Fuller, A.; Maloney, S. K.; Dell'Omo, G.; Vyssotski, A. L.; Rattenborg, N. C. (2011). Balaban, Evan, ed. "Ostriches Sleep like Platypuses". PLoS ONE. 6 (8): e23203. doi:10.1371/journal.pone.0023203. PMC 3160860
 . PMID 21887239.
. PMID 21887239. - ↑ Rechtschaffen, A; Kales, A (1968). A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. US Dept of Health, Education, and Welfare; National Institutes of Health.
- ↑ Carlson, Neil R. (2012). Physiology of Behavior. Pearson. p. 291. ISBN 0205239390.
- ↑ Schulz, Hartmut (2008). "Rethinking sleep analysis. Comment on the AASM Manual for the Scoring of Sleep and Associated Events". J Clin Sleep Med. American Academy of Sleep Medicine. 4 (2): 99–103. PMC 2335403
 . PMID 18468306.
. PMID 18468306. Although the sequence of non-REM (NREM) sleep stages one to four (R&K classification) or N1 to N3 (AASM classification) fulfills the criteria...
- ↑ "Glossary. A resource from the Division of Sleep Medicine at Harvard Medical School, Produced in partnership with WGBH Educational Foundation". Harvard University. 2008. Retrieved 2009-03-11.
The 1968 categorization of the combined Sleep Stages 3 - 4 was reclassified in 2007 as Stage N3.
- 1 2 Iber, C; Ancoli-Israel, S; Chesson, A; Quan, SF. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: American Academy of Sleep Medicine; 2007.
- ↑ Carlson, Neil R. (2012). Physiology of Behavior. Pearson. p. 291,293. ISBN 0205239390.
- ↑ http://www.nytimes.com/2013/01/28/health/brain-aging-linked-to-sleep-related-memory-decline.html
- ↑ http://walkerlab.berkeley.edu/reprints/Walker_JCSM_2009.pdf
- 1 2 http://www.architalbiol.org/aib/article/viewFile/411/370
- 1 2 3 4 Carlson, Neil R. (2012). Physiology of Behavior. Pearson. pp. 297–298. ISBN 0205239390.
- 1 2 3 Slow-Wave Sleep: Beyond Insomnia. Wolters Kluwer Pharma Solutions. ISBN 978-0-9561387-1-2.
- ↑ McGinty, Dennis; Ronald Szymusiak (1990). "Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep". Science Direct. Trends in Neuroscience. 13 (12): 480–487. doi:10.1016/0166-2236(90)90081-k.
- ↑ Afaghi A, O'Connor H, Chow CM (August 2008). "Acute effects of the very low carbohydrate diet on sleep indices". Nutr Neurosci. 11 (4): 146–54. doi:10.1179/147683008X301540. PMID 18681982.
- ↑ Schierenbeck, T.; Riemann, D.; Berger, M.; Hornyak, M. (Oct 2008). "Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana". Sleep Med Rev. 12 (5): 381–389. doi:10.1016/j.smrv.2007.12.004. PMID 18313952.
- ↑ Gates, P.J.; Albertella, L.; Copeland, J. (Feb 2014). "The effects of cannabinoid administration on sleep: a systematic review of human studies". Sleep Med Rev. 18: 477–487. doi:10.1016/j.smrv.2014.02.005. PMID 24726015.
- ↑ http://www.theodora.com/drugs/eu/xyrem.html
- ↑ http://www.brighamandwomens.org/about_bwh/publicaffairs/news/pressreleases/PressRelease.aspx?sub=0&PageID=942
- ↑ http://www.massgeneral.org/about/pressrelease.aspx?id=1502
- ↑ Carlson, Neil R. (2012). Physiology of Behavior. Pearson. p. 305-307. ISBN 0205239390.
- ↑ http://www.hgi.org.uk/archive/sleepanddream1.htm#.U2kpm8fhjys
- ↑ Carlson, Neil R. (2012). Physiology of Behavior. Pearson. p. 299-300. ISBN 0205239390.
- ↑ Carlson, Neil R. (2012). Physiology of Behavior. Pearson. p. 298. ISBN 0205239390.
- ↑ http://onlinelibrary.wiley.com/enhanced/doi/10.1111/j.1365-2869.2011.00959.x/
- ↑ Williams SR, Tóth TI, Turner JP, Hughes SW, Crunelli W (1997) The window component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol 505:689–705.
Further reading
- M. Massimini, G. Tononi, et al., "Breakdown of Cortical Effective Connectivity During Sleep," Science, vol. 309, 2005, pp. 2228–32.
- P. Cicogna, V. Natale, M. Occhionero, and M. Bosinelli, "Slow Wave and REM Sleep Mentation," Sleep Research Online, vol. 3, no. 2, 2000, pp. 67–72.
- D. Foulkes et al., "Ego Functions and Dreaming During Sleep Onset," in Charles Tart, ed., Altered States of Consciousness, p. 75.
- Rock, Andrea (2004). The Mind at Night.
- Warren, Jeff (2007). "The Slow Wave". The Head Trip: Adventures on the Wheel of Consciousness. ISBN 978-0-679-31408-0.
