Sevelamer
 | |
| Clinical data | |
|---|---|
| Trade names | Renagel, Renvela |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601248 |
| Pregnancy category | |
| Routes of administration | oral |
| ATC code | V03AE02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | nil |
| Metabolism | nil |
| Biological half-life | n/a |
| Excretion | faecal 100% |
| Identifiers | |
| |
| CAS Number |
52757-95-6 |
| PubChem (CID) | 3085017 |
| DrugBank |
DB00658 |
| ChemSpider |
2341997 |
| UNII |
941N5DUU5C |
| KEGG |
D08512 |
| ChEMBL |
CHEMBL1201492 |
| ECHA InfoCard | 100.207.865 |
| Chemical and physical data | |
| Formula |
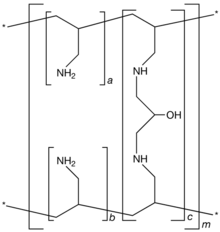
[(C3H7N)a+b.(C9H17N2O)c]m where a+b:c = 9:1 |
| Molar mass | variable |
| | |
Sevelamer (rINN) (/sɛˈvɛləmər/ or /sɛˈvɛləmɪər/) is a phosphate binding drug used to treat hyperphosphatemia in patients with chronic kidney disease. When taken with meals, it binds to dietary phosphate and prevents its absorption. Sevelamer was invented and developed by GelTex Pharmaceuticals. Sevelamer is currently marketed by Sanofi under the trade names Renagel (sevelamer hydrochloride) and Renvela (sevelamer carbonate).
Chemistry and pharmacology
Sevelamer consists of polyallylamine that is crosslinked with epichlorohydrin.[1] The marketed form sevelamer hydrochloride is a partial hydrochloride salt being present as approximately 40% amine hydrochloride and 60% sevelamer base. The amine groups of sevelamer become partially protonated in the intestine and interact with phosphate ions through ionic and hydrogen bonding.
Clinical use
Indications
Sevelamer is indicated for the management of hyperphosphataemia in adult patients with stage 4 and 5 chronic kidney disease on hemodialysis.
Contraindications
Sevelamer therapy is contraindicated in hypophosphataemia or bowel obstruction.
Adverse effects
Common adverse drug reactions (ADRs) associated with the use of sevelamer include: hypotension, hypertension, nausea and vomiting, dyspepsia, diarrhea, flatulence, and/or constipation.
Other effects
Sevelamer can significantly reduce serum uric acid.[2] This reduction has no known detrimental effect and several beneficial effects, including reducing hyperuricemia, uric acid nephrolithiasis, and gout.
References
- ↑ (1) Rosenbaum, D. P.; Mandeville, W. H.; Pitruzzello, M.; Goldberg, D. I. Nephrology Dialysis Transplantation Effect of RenaGel A , a Non-Absorbable , Cross-Linked , Polymeric Phosphate Binder , on Urinary Phosphorus Excretion in Rats. 1997, 961–964.
- ↑ Garg JP, Chasan-Taber S, Blair A, et al. (January 2005). "Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial". Arthritis and rheumatism. 52 (1): 290–5. doi:10.1002/art.20781. PMID 15641045.