Sea urchin
| Sea urchin Temporal range: Ordovician–Recent | |
|---|---|
_and_Echinometra_viridis_(Reef_Urchin_-_bottom).jpg) | |
| Tripneustes ventricosus and Echinometra viridis, two species of tropical sea urchins. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Echinodermata |
| Subphylum: | Echinozoa |
| Class: | Echinoidea Leske, 1778 |
| Subclasses | |
| |
Sea urchins or urchins (/ˈɜːrtʃɪnz/), archaically called sea hedgehogs,[1][2] are small, spiny, globular animals that, with their close kin, such as sand dollars, constitute the class Echinoidea of the echinoderm phylum. About 950 species of echinoids inhabit all oceans from the intertidal to 5,000 metres (16,000 ft; 2,700 fathoms) deep.[3] The shell, or "test", of sea urchins is round and spiny, typically from 3 to 10 cm (1.2 to 3.9 in) across. Common colors include black and dull shades of green, olive, brown, purple, blue, and red. Sea urchins move slowly, feeding primarily on algae. Sea otters, starfish, wolf eels, triggerfish, and other predators hunt and feed on sea urchins. Their roe is a delicacy in many cuisines. The name "urchin" is an old word for hedgehog, which sea urchins resemble.
Taxonomy

Sea urchins are members of the phylum Echinodermata, which also includes sea stars, sea cucumbers, brittle stars, and crinoids. Like other echinoderms, they have five-fold symmetry (called pentamerism) and move by means of hundreds of tiny, transparent, adhesive "tube feet". The symmetry is not obvious in the living animal, but is easily visible in the dried test.
Specifically, the term "sea urchin" refers to the "regular echinoids", which are symmetrical and globular, and includes several different taxonomic groups, including two subclasses : Euechinoidea ("modern" sea urchins, including irregular ones) and Cidaroidea or "slate-pencil urchins", which have very thick, blunt spines, with algae and sponges growing on it. The irregular sea urchins are an infra-class inside the Euechinoidea, called Irregularia, and include Atelostomata and Neognathostomata. "Irregular" echinoids include: flattened sand dollars, sea biscuits, and heart urchins.
Together with sea cucumbers (Holothuroidea), they make up the subphylum Echinozoa, which is characterized by a globoid shape without arms or projecting rays. Sea cucumbers and the irregular echinoids have secondarily evolved diverse shapes. Although many sea cucumbers have branched tentacles surrounding their oral openings, these have originated from modified tube feet and are not homologous to the arms of the crinoids, sea stars, and brittle stars.
-
Paracentrotus lividus, a regular sea urchin (Euechinoidea, infraclass Carinacea).
-

A sand dollar, an irregular sea urchin (Irregularia).
-

Phyllacanthus imperialis, a cidaroid sea urchin (Cidaroidea).
Anatomy

Urchins typically range in size from 6 to 12 cm (2.4 to 4.7 in), although the largest species can reach up to 36 cm (14 in).[4]
Fivefold symmetry
Like other echinoderms, sea urchin early larvae have bilateral symmetry,[5] but they develop five-fold symmetry as they mature. This is most apparent in the "regular" sea urchins, which have roughly spherical bodies with five equally sized parts radiating out from their central axes. Several sea urchins, however, including the sand dollars, are oval in shape, with distinct front and rear ends, giving them a degree of bilateral symmetry. In these urchins, the upper surface of the body is slightly domed, but the underside is flat, while the sides are devoid of tube feet. This "irregular" body form has evolved to allow the animals to burrow through sand or other soft materials.[4]
Organs and test
The lower half of a sea urchin's body is referred to as the oral surface, because it contains the mouth, while the upper half is the aboral surface. The internal organs are enclosed in a hard shell or "test" composed of fused plates of calcium carbonate covered by a thin dermis and epidermis. The test is rigid, and divides into five ambulacral grooves separated by five interambulacral areas. Each of these areas consists of two rows of plates, so the sea urchin test includes 20 rows of plates in total. The plates are covered in rounded tubercles, to which the spines are attached. The inner surface of the test is lined by peritoneum.[4] Sea urchins convert aqueous carbon dioxide using a catalytic process involving nickel into the calcium carbonate portion of the test.[6]
Feet
Sea urchins' tube feet arise from the five ambulacral grooves. Tube feet are moved by a water vascular system, which works through hydraulic pressure, allowing the sea urchin to pump water into and out of the tube feet, enabling it to move.
.jpg)
Mouth/anus
The mouth lies in the centre of the oral surface in regular urchins, or towards one end in irregular urchins. It is surrounded by lips of softer tissue, with numerous small, bony pieces embedded in it. This area, called the peristome, also includes five pairs of modified tube feet and, in many species, five pairs of gills. On the upper surface, opposite the mouth, is the periproct, which surrounds the anus. The periproct contains a variable number of hard plates, depending on species, one of which contains the madreporite.[4] The structure of the mouth and teeth have been found to be so efficient at grasping and grinding that their structure has been tested for use in real-world applications.[7]
Endoskeleton
The sea urchin builds its spicules, the sharp, crystalline "bones" that constitute the animal’s endoskeleton, in the larval stage. The fully formed spicule is composed of a single crystal with an unusual morphology. It has no facets, and within 48 hours of fertilization, assumes a shape that looks much like the Mercedes-Benz logo.[8]
In other echinoderms, the endoskeleton is associated with a layer of muscle that allows the animal to move its arms or other body parts. This is entirely absent in sea urchins, which are unable to move in this way.
-

Test of an Echinus esculentus, a regular sea urchin.
-

Test of an Echinodiscus tenuissimus, an irregular sea urchin ("sand dollar").
-
Test of a Phyllacanthus imperialis, a cidaroid sea urchin. These are characterised by their big tubercles, bearing large radiola.
-
.jpg)
Close-up on the test of a regular sea urchin. One can see an ambulacrum (yellow) with its two rows of pore-pairs, between two interambulacra (green). The tubercles are non perforated.
-

Close-up on a cidaroid sea urchin apical disc : the 5 holes are the gonopores, and the central one is the anus ("periproct"). The biggest genital plate is the madreporite.
Spines

Typical sea urchins have spines about 1 to 3 cm (0.39 to 1.18 in) in length, 1 to 2 mm (0.039 to 0.079 in) thick, and more or less sharp. The genus Diadema, familiar in the tropics, has the longest spines; they are thin and can reach 10 to 30 cm (3.9 to 11.8 in) long.
The spines, long and sharp in some species, protect the urchin from predators. They inflict a painful wound when they penetrate human skin, but are not themselves dangerous if fully removed promptly; if left in the skin, further problems may occur.[9]
Some families of tropical sea urchins are known to have venomous spines, like Diadematidae and Echinothuriidae. The first family contains the "Diadem" sea urchins, and the latter the "fire urchins".
Many urchins in the Toxopneustidae are venomous as well, but the danger does not come from their spines (short and blunt) but from their pedicellariae, like the collector urchin and especially the flower urchin, the only potentially lethal echinoderm known to date.[10]
Reproductive organs
.theora.ogv.jpg)
Sea urchins are dioecious, having separate male and female sexes, although distinguishing the two is not easy, except for their locations on the sea bottom. Males generally choose elevated and exposed locations, so their milt can be broadcast by sea currents. Females generally choose low-lying locations in sea bottom crevices, presumably so the tiny larvae can have better protection from predators. Indeed, very small sea urchins are found hiding beneath rocks. Regular sea urchins have five gonads, lying underneath the interambulacral regions of the test, while the irregular forms have only four, with the hindmost gonad being absent. Each gonad has a single duct rising from the upper pole to open at a gonopore lying in one of the genital plates surrounding the anus. The gonads are lined with muscles underneath the peritoneum, and these allow the animal to squeeze its gametes through the duct and into the surrounding sea water where fertilization takes place.[4]
Physiology
Digestion
The mouth of most sea urchins is made up of five calcium carbonate teeth or jaws, with a fleshy, tongue-like structure within. The entire chewing organ is known as Aristotle's lantern (image), from Aristotle's description in his History of Animals:
- ...the urchin has what we mainly call its head and mouth down below, and a place for the issue of the residuum up above. The urchin has, also, five hollow teeth inside, and in the middle of these teeth a fleshy substance serving the office of a tongue. Next to this comes the esophagus, and then the stomach, divided into five parts, and filled with excretion, all the five parts uniting at the anal vent, where the shell is perforated for an outlet... In reality the mouth-apparatus of the urchin is continuous from one end to the other, but to outward appearance it is not so, but looks like a horn lantern with the panes of horn left out. (Tr. D'Arcy Thompson)
However, this has recently been proven to be a mistranslation. Aristotle's lantern is actually referring to the whole shape of sea urchins, which look like the ancient lamps of Aristotle's time.[11][12]
Heart urchins are unusual in not having a lantern. Instead, the mouth is surrounded by cilia that pull strings of mucus containing food particles towards a series of grooves around the mouth.[4]
The lantern, where present, surrounds both the mouth cavity and the pharynx. At the top of the lantern, the pharynx opens into the esophagus, which runs back down the outside of the lantern, to join the small intestine and a single caecum. The small intestine runs in a full circle around the inside of the test, before joining the large intestine, which completes another circuit in the opposite direction. From the large intestine, a rectum ascends towards the anus. Despite the names, the small and large intestines of sea urchins are in no way homologous to the similarly named structures in vertebrates.[4]
Digestion occurs in the intestine, with the caecum producing further digestive enzymes. An additional tube, called the siphon, runs beside much of the intestine, opening into it at both ends. It may be involved in resorption of water from food.[4]
Circulation
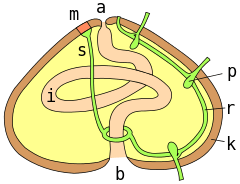
a = anus ; m = madreporite ; s = aquifer canal ; r = radial canal ; p = podial ampulla ; k = test wall ; i = intestine ; b = mouth.
Sea urchins possess both a water vascular system and a hemal system, the latter containing blood. However, the main circulatory fluid fills the general body cavity, or coelom. This fluid contains phagocytic coelomocytes, which move through the vascular and hemal systems. The coelomocytes are an essential part of blood clotting, but also collect waste products and actively remove them from the body through the gills and tube feet.[4]
Respiration
Most sea urchins possess five pairs of external gills, located around their mouths. These thin-walled projections of the body cavity are the main organs of respiration in those urchins that possess them. Fluid can be pumped through the gills' interiors by muscles associated with the lantern, but this is not continuous, and occurs only when the animal is low on oxygen. Tube feet can also act as respiratory organs, and are the primary sites of gas exchange in heart urchins and sand dollars, both of which lack gills.[4]
Nervous system
The nervous system of sea urchins has a relatively simple layout. With no true brain, the neural center is a large nerve ring encircling the mouth just inside the lantern. From the nerve ring, five nerves radiate underneath the radial canals of the water vascular system, and branch into numerous finer nerves to innervate the tube feet, spines, and pedicellariae.[4]
Senses
Sea urchins are sensitive to touch, light, and chemicals. Although they do not have eyes or eye spots (except for diadematids, which can follow a threat with their spines), the entire body of most regular sea urchins might function as a compound eye.[13] They also have statocysts, called spheridia, located within the ambulacral plates to help the animal remain upright.[4]
Development
Ingression of primary mesenchyme cells

During early development, the sea urchin embryo undergoes 10 cycles of cell division,[14] resulting in a single epithelial layer enveloping a blastocoel.[15] The embryo must then begin gastrulation, a multipart process which involves the dramatic rearrangement and invagination of cells to produce the three germ layers.
The first step of gastrulation is the epithelial-to-mesenchymal transition and ingression of primary mesenchyme cells into the blastocoel.[15] Primary mesenchyme cells, or PMCs, are located in the vegetal plate specified to become mesoderm.[16] Prior to ingression, PMCs exhibit all the features of other epithelial cells that comprise the embryo. Cells of the epithelium are bound basally to a laminal matrix and apically to an extraembryonic matrix.[16] The apical microvilli of these cells reach into the hyaline layer, a component of the extraembryonic matrix.[17] Neighboring epithelial cells are also connected to each other through apical junctions,[18] protein complexes containing adhesion molecules, such as cadherins, linked to catenins.


As PMCs begin to undergo an epithelial-to-mesenchymal transition, the lamina which binds them dissolves to begin the mechanical release of the cells.[17] Expression of the membrane protein that binds laminin, integrin, also becomes irregular at the beginning of ingression.[19] The microvilli which secure PMCs to the hyaline layer shorten,[20] as the cells reduce their affinity for the extraembryonic matrix.[21] These cells concurrently increase their affinity for other components of the basal matrix, such as fibronectin, in part driving the movement of cells inward.[21] The apical junctions which bind PMCs to their neighboring epithelial cells become disrupted during this transition, and are absent in cells that have fully ingressed into the blastocoel.[17] Because staining for cadherins and catenins in ingressing cells decreases and develops as intracellular accumulations, apical junctions are thought to be cleared by endocytosis during ingression.[22][23]
Once the PMCs disrupt all attachment to their former location, the cells themselves change their morphology by contracting their apical surfaces, apical constriction, and enlarging their basal surfaces, thus acquiring a "bottle cell" phenotype.[16] Cytoskeletal rearrangements mediate the shape changes of PMCs; though the cytoskeleton assists in the mechanics of ingression, other mechanisms drive the process. Experimentally disrupting microtubule dynamics in the species Strongylocentrotus pupuratus by applying colchicine stalls the ingression of PMCs, but does not inhibit it.[24] Similarly, experimentally disrupting actin-myosin contraction using inhibitors slows down ingression, but does not arrest the process.[25]
The morphogenetic movements of the PMCs are an autonomous cellular behavior. Experimentally grafting PMCs into heterotopic tissue does not prevent the cells from ingressing.[21] In studies where PMCs are cultured in insolation, the cells were observed to gain affinity for fibronectin and simultaneously lose affinity for extraembryonic matrix, independent of the embryonic environment.[21]

Life history
At first glance, sea urchins often appear incapable of moving. Sometimes, the most visible life sign is the spines, which attach to ball-and-socket joints and can point in any direction. In most urchins, touch elicits a prompt reaction from the spines, which converge toward the touch point. Sea urchins have no visible eyes, legs, or means of propulsion, but can move freely over hard surfaces using adhesive tube feet, working in conjunction with the spines. Regular sea urchins don't have any favourite walking direction.[26]
Reproduction
In most cases, the female's eggs float freely in the sea, but some species hold onto them with their spines, affording them a greater degree of protection. The fertilized egg, once met with the free-floating sperm released by males, develops into a free-swimming blastula embryo in as few as 12 hours. Initially a simple ball of cells, the blastula soon transforms into a cone-shaped echinopluteus larva. In most species, this larva has 12 elongated arms lined with bands of cilia that capture food particles and transport them to the mouth. In a few species, the blastula contains supplies of nutrient yolk and lacks arms, since it has no need to feed.[4]
Several months are needed for the larva to complete its development, which begins with the formation of the test plates around the mouth and anus. Soon, the larva sinks to the bottom and metamorphoses into adult form in as quickly as one hour. In some species, adults reach their maximum size in about five years.[4]
Predators

Adult sea urchins are usually well protected against most predators by their strong and sharp spines, which can be poisonous in some species,[27] but when they are damaged they quickly attract lots of fish and other omnivorous animals.
Sea urchins are one of the favourite food of many lobsters, crabs, triggerfish, California sheephead, sea otter and wolf eels : all these animals carry particular adaptations (teeth, pincers, claws) and a strength that allow them to overcome the excellent protective features of sea urchins.
Sea urchins can also be infected by many parasites, be they internal or external. Pedicellaria[28] are a good means of defense against ectoparasites, but not a panacea as some of them actually feed on it.[29] The hemal system ensures against endoparasites.
-

A sea otter feeding on a purple sea urchin.
-

A crab (Carpilius convexus) attacking a slate pencil sea urchin (Heterocentrotus mamillatus).
-

A wrasse finishing the remains of a damaged Tripneustes gratilla.
Ecology

Sea urchins feed mainly on algae but can feed on sea cucumbers and a wide range of invertebrates, such as mussels, polychaetes, sponges, brittle stars, and crinoids.[30] Population densities vary by habitat, with more dense populations in barren areas as compared to kelp stands.[31][32] Even in these barren areas, greatest densities are found in shallow water. Populations are generally found in deeper water if wave action is present.[32] Densities decrease in winter when storms cause them to seek protection in cracks and around larger underwater structures.[32]
Sea urchins are some of the favorite foods of sea otters and California sheephead, and are the main source of nutrition for wolf eels. Left unchecked, urchins devastate their environments, creating what biologists call an urchin barren, devoid of macroalgae and associated fauna. Sea otters have re-entered British Columbia, dramatically improving coastal ecosystem health.[33]
Range and habitat


Sea urchins have conquered most sea habitats, on an extremely wide range of depths.[34] Some species, such as Cidaris abyssicola, can live down to several thousands of meters deep. Many genera are totally indentured to the abyssal zone, such as many cidaroids, most of the genera in the Echinothuriidae family, or the strange genus Dermechinus. One of the deepest-living family is the Pourtalesiidae,[35] strange bottle-shaped irregular sea urchins that live only in the hadal zone and have been collected as far as 6850 meters deep in the Java trench.[36] Nevertheless, this makes sea urchin the class of echinoderms living the least deep, compared to sea cucumbers and crinoids that remain abundant below 8000 m.[36]
Sea urchins can be found in all climates, from warm seas to polar oceans [34] (like the polar sea urchin Sterechinus neumayeri). They adapt their diet to their environment: In rich ecosystems they feed mainly on algae that allow a quick growth; in less rich bottoms they adopt a slower metabolism, adapted to a less calorific diet.
The shingle urchin (Colobocentrotus atratus), which lives on exposed shorelines, is particularly resistant to wave action. It is one of the few sea urchin that can survive many hours out of water.[37]
Despite their presence in nearly all the marine ecosystems, most species are encountered on temperate and tropical coasts, between the surface and some tens of meters deep, close to photosynthetic food sources.[34]
-

Purple sea urchins at low tide in California. They dig a cavity in the rock to hide from predators during the day.
-

"Sand dollars" live in the sediment, and feed on it.
-

Dermechinus horridus, an abyssal species, at thousands of meters deep.
Evolutionary history
The earliest echinoid fossils date to the upper part of the Ordovician period (circa 450 Mya), and the taxon has survived to the present as a successful and diverse group of organisms. Spines may be present in well-preserved specimens, but usually only the test remains. Isolated spines are common as fossils. Some echinoids (such as Tylocidaris clavigera, from the Cretaceous period's English Chalk Formation) had very heavy, club-shaped spines that would be difficult for an attacking predator to break through and make the echinoid awkward to handle. Such spines simplify walking on the soft sea floor.

Most of the fossil echinoids from the Paleozoic era are incomplete, consisting of isolated spines and small clusters of scattered plates from crushed individuals, mostly in Devonian and Carboniferous rocks. The shallow-water limestones from the Ordovician and Silurian periods of Estonia are famous for echinoids. Paleozoic echinoids probably inhabited relatively quiet waters. Because of their thin tests, they would certainly not have survived in the wave-battered coastal waters inhabited by many modern echinoids. During the upper part of the Carboniferous period, a marked decline in echinoid diversity occurred, and this trend continued to the Permian period. They neared extinction at the end of the Paleozoic era, with just six species known from the Permian period. Only two lineages survived this period's massive extinction and into the Triassic: the genus Miocidaris, which gave rise to modern cidaroida (pencil urchins), and the ancestor that gave rise to the euechinoids. By the upper part of the Triassic period, their numbers began to increase again. Cidaroids have changed very little since the Late Triassic and are today considered to be living fossils. Diversity of echinoids have been fluctuating across the eons. Among the different proxies, marine facies variation in combination with outcrop area best explains the palaeodiversity curve.[38]

The euechinoids, though, diversified into new lineages throughout the Jurassic and into the Cretaceous periods, and from them emerged the first irregular echinoids (superorder Atelostomata) during the early Jurassic, and later the other superorder (Gnathostomata) of irregular urchins, which evolved independently. These superorders today represent 47% of all extant species of echinoids because of their adaptive breakthroughs, which allowed them to exploit habitats and food sources unavailable to regular echinoids. During the Mesozoic and Cenozoic eras, the echinoids flourished. Most echinoid fossils are often abundant in the restricted localities and formations where they occur. An example of this is Enallaster, which exists by the thousands in certain outcrops of limestone from the Cretaceous period in Texas. Many fossils of the Late Jurassic Plesiocidaris still have the spines attached.
Some echinoids, such as Micraster, which is found in the Cretaceous period Chalk Formation of England and France, serve as zone or index fossils. Because they evolved rapidly, they aid geologists in dating the surrounding rocks. However, most echinoids are not abundant enough and are of too limited range to serve as zone fossils.
In the Paleogene and Neogene periods (circa 66 to 1.8 Mya), sand dollars (order Clypeasteroida) arose. Their distinctive, flattened tests and tiny spines were adapted to life on or under loose sand. They form the newest branch on the echinoid tree.


Phylogeny
- Sub-class Euechinoidea Bronn, 1860
- Infra-class Acroechinoidea Smith, 1981
- Order Aspidodiadematoida Kroh & Smith, 2010
- Order Diadematoida Duncan, 1889
- Order Micropygoida Kroh & Smith, 2010
- Order Pedinoida Mortensen, 1939
- Infra-class Carinacea Kroh & Smith, 2010
- Super-order Calycina Gregory, 1900
- Order Phymosomatoida Mortensen, 1904 †
- Order Salenioida Delage & Hérouard, 1903
- Super-order Echinacea Claus, 1876
- Order Arbacioida Gregory, 1900
- Order Camarodonta Jackson, 1912
- Order Stomopneustoida Kroh & Smith, 2010
- Super-order Calycina Gregory, 1900
- Order Echinothurioida Claus, 1880
- Infra-class Irregularia Latreille, 1825
- Super-order Atelostomata von Zittel, 1879
- Order Holasteroida Durham & Melville, 1957
- Order Spatangoida L. Agassiz, 1840a
- Order Echinoneoida H. L. Clark, 1925
- Order Holectypoida Ducan, 1889 †
- Super-order Neognathostomata Smith, 1981
- Order Cassiduloida Claus, 1880
- Order Clypeasteroida A. Agassiz, 1872
- Order Echinolampadoida Kroh & Smith, 2010
- Order Nucleolitidae L. Agassiz & Desor, 1847 †
- Super-order Atelostomata von Zittel, 1879
- Infra-class Acroechinoidea Smith, 1981
- Sub-class Cidaroidea Smith, 1984
- Order Cidaroida Claus, 1880
-
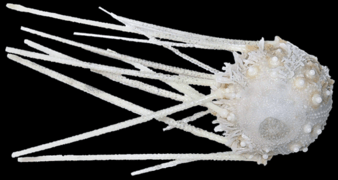
Salenocidaris hastigera (dried), a Salenioida
-
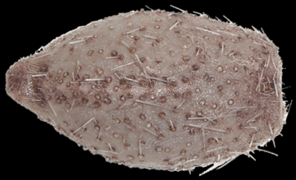
Pourtalesia wandeli (dried), a Holasteroida
Relation to humans
"Stings" to humans
Sea urchin puncture wounds are a common source of injury to ocean swimmers, especially along coastal surfaces where coral with stationary sea urchins are present. Sea urchin stings vary in severity depending on the species. When handling sea urchins for cleaning in meal preparation, laborers emphasize that there is a technique: "The key is to not grab them firmly and too fast." Handling them usually requires a pair of thick fabric gloves and rubber gloves over top of them; however, this combination is not completely impenetrable, and you can still be stung.[41]
In Biological Research
Sea urchins are traditional model organisms in developmental biology. This use originated in the 1800s, when their embryonic development became easily viewed by microscopy. The transparency of the urchin's eggs enabled them to be used to observe that sperm cells actually fertilize ova.[42] The urchin was also very accesible to early researchers because of its bilaterally organized embryo. [43]
The more recent resurgence for the organism has come from two scientists in particular, Eric H. Davidson and Roy John Britten. Over 30 years ago the two men stationed at the California Institute of Technology began pushing for the use of urchins as a model organism due to their easy availability, high fecundity, long lifespan, and substantial usefulness in observation of a variety of tasks.[44] Beyond the study of Embryo development, urchins allow for the opportunity to research the structure and function of cis-regulation (see Cis-regulatory element).[45] The organism's evolutionary placement and functional advanatages were the major arguements in the proposal to seek the sequencing of its species' genome. A wholly modern discovery for sequencing the urchin genomes came in the realization that urchins act as the closest living relative to chordates. [46]
The sequencing of the whole genome of a certain sea urchin genome,Strongylocentrotus purpuratus, was completed in 2006 and established homology between sea urchin and vertebrate immune system-related genes. Sea urchins code for at least 222 toll-like receptor genes and over 200 genes related to the nod-like-receptor family found in vertebrates.[47] This increases its usefulness as a valuable model organism for studying the evolution of innate immunity. The sequencing also revealed that while some things were thought to be strictly vertebrate characteristics, there were also innovations that have previously only been seen outside the Deuterostome classification. [48]
Since the sequencing of Strongylocentrotus purpuratus and several other species of urchins, urchins have been used for research applications in studies in a multitude of ways. They continue to be utilized for embryonic studies, as Prenatal development continues to seek testing for fatal diseases. Sea urchins are being used in longevity studies for comparison between the young and old of the species, particularly for their ability to regenerate tissue as needed.[49] Scientists at the University of St Andrews have discovered a genetic sequence in sea urchins previously thought to have belonged only to certain viruses that afflict humans. [50] Oceanography has taken in interest in monitoring the health of urchins and their populations as a way to assess overall ocean acidification[51], temperatures, and ecological impact studies.

As food

The gonads of both male and female sea urchins, usually called sea urchin roe or corals,[52] are culinary delicacies in many parts of the world.[53][54]
In cuisines around the Mediterranean, Paracentrotus lividus is often eaten raw, or with lemon,[55] and known as ricci on Italian menus where it is sometimes used in pasta sauces. It can also flavour omelettes, scrambled eggs, fish soup,[56] mayonnaise, béchamel sauce for tartlets,[57] the boullie for a soufflé,[58] or Hollandaise sauce to make a fish sauce.[59] In Chilean cuisine, it is served raw with lemon, onions, and olive oil.
Though the edible Strongylocentrotus droebachiensis is found in the North Atlantic, it is not widely eaten. However, sea urchins (called uutuk in Alutiiq) are commonly eaten by the Alaska Native population around Kodiak Island. It is commonly exported, mostly to Japan.[60]
In the West Indies, slate pencil urchins are eaten.[61]
On the Pacific Coast of North America, Strongylocentrotus franciscanus was praised by Euell Gibbons; Strongylocentrotus purpuratus is also eaten.
In New Zealand, Evechinus chloroticus, known as kina in Maori, is a delicacy, traditionally eaten raw. Though New Zealand fishermen would like to export them to Japan, their quality is too variable.[62]
In Japan, sea urchin is known as uni (うに), and its roe can retail for as much as A$450/kg;[63] it is served raw as sashimi or in sushi, with soy sauce and wasabi. Japan imports large quantities from the United States, South Korea, and other producers. Japanese demand for sea urchin corals has raised concerns about overfishing.[64]
Native Americans in California are also known to eat sea urchins.[65]
Aquaria
Some species of sea urchins, such as the slate pencil urchin (Eucidaris tribuloides), are commonly sold in aquarium stores. Some species are effective at controlling hair algae, and they make good additions to an invertebrate tank.
Bibliography
- Smith, Andrew B. (1984), Echinoid Palaeobiology (Special topics in palaeontology). London: Allen & Unwin. ISBN 0-04-563001-1
- Schultz, Heinke. (2005), Sea-Urchins, a guide to worldwide shallow water species . hpsp scientific publications, Germany. ISBN 3-9809868-1-0
- Animal Diversity Web Classification of the Echinoidea
- Ocean Alliance giving advice on sea urchin cleaning
See also
References
- ↑ Wright, Anne. 1851. The Observing Eye, Or, Letters to Children on the Three Lowest Divisions of Animal Life. London: Jarrold and Sons, p. 107.
- ↑ Soyer, Alexis. 1853. The Pantropheon Or History Of Food, And Its Preparation: From The Earliest Ages Of The World. Boston: Ticknor, Reed, and Fields,, p. 245.
- ↑ "Animal Diversity Web – Echinoidea". University of Michigan Museum of Zoology. Retrieved 2012-08-26.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 961–981. ISBN 0-03-056747-5.
- ↑ Stachan and Read, Human Molecular Genetics, p. 381: "What Makes Us Human"
- ↑ "Sea urchins reveal promising carbon capture alternative". Gizmag. 4 February 2013. Retrieved 2013-02-05.
- ↑ Claw inspired by sea urchins' mouth can scoop up Martian soil
- ↑ Balakirev, E. S.; Pavlyuchkov, V. A.; Ayala, F. J. (2008). "DNA variation and symbiotic associations in phenotypically diverse sea urchin Strongylocentrotus intermedius". Proceedings of the National Academy of Sciences. 105 (42): 16218–16223. Bibcode:2008PNAS..10516218B. doi:10.1073/pnas.0807860105. Lay summary – University of Wisconsin-Madison (October 27, 2008).
- ↑ Matthew D. Gargus; David K. Morohashi (2012). "A sea-urchin spine chilling remedy" (letter to the Editor). New England Journal of Medicine. 30 (19): 1867–1868. doi:10.1056/NEJMc1209382.
- ↑ Mah, Christopher (February 5, 2014). "What we know about the world's most venomous sea urchin Toxopneustes fits in this blog post!". The Echinoblog..
- ↑ Voultsiadou, Eleni; Chintiroglou, Chariton (2008). "Aristotle's lantern in echinoderms: an ancient riddle". Cahiers de Biologie Marine. Station Biologique de Roscoff. 49 (3): 299–302.
- ↑ http://news.nationalgeographic.com/news/2010/12/101228-sea-urchin-teeth-self-sharpening-tools-science-animals/, National Geographic: "Rock-Chewing Sea Urchins Have Self-Sharpening Teeth."
- ↑ Knight, K. (2009). "Sea Urchins Use Whole Body As Eye". Journal of Experimental Biology. 213 (2): i. doi:10.1242/jeb.041715. Lay summary – LiveScience (December 28, 2009).
- ↑ A. Gaion, A. Scuderi; D. Pellegrini; D. Sartori (2013). "Arsenic Exposure Affects Embryo Development of Sea Urchin, Paracentrotus lividus (Lamarck, 1816)". Bulletin of Environmental Contamination and Toxicology: 1–5. doi:10.3109/01480545.2015.1041602.
- 1 2 Kominami, Tetsuya; Takata, Hiromi (2004). "Gastrulation in the sea urchin embryo: a model system for analyzing the morphogenesis of a monolayered epithelium". Development, growth & differentiation. 46 (4): 309–26. doi:10.1111/j.1440-169x.2004.00755.x.
- 1 2 3 Shook, D; Keller, R (2003). "Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development". Mechanisms of development. 120 (11): 1351–83. doi:10.1016/j.mod.2003.06.005. PMID 14623443.
- 1 2 3 Katow, Hideki; Solursh, Michael (1980). "Ultrastructure of primary mesenchyme cell ingression in the sea urchinLytechinus pictus". Journal of Experimental Zoology. 213 (2): 231–246. doi:10.1002/jez.1402130211.
- ↑ Balinsky, BI (1959). "An electro microscopic investigation of the mechanisms of adhesion of the cells in a sea urchin blastula and gastrula". Experimental Cell Research. 16 (2): 429–33. doi:10.1016/0014-4827(59)90275-7. PMID 13653007.
- ↑ Hertzler, PL; McClay, DR (1999). "alphaSU2, an epithelial integrin that binds laminin in the sea urchin embryo". Developmental Biology. 207 (1): 1–13. doi:10.1006/dbio.1998.9165. PMID 10049560.
- ↑ Fink, RD; McClay, DR (1985). "Three cell recognition changes accompany the ingression of sea urchin primary mesenchyme cells". Developmental Biology. 107 (1): 66–74. doi:10.1016/0012-1606(85)90376-8. PMID 2578117.
- 1 2 3 4 Burdsal, CA; Alliegro, MC; McClay, DR (1991). "Tissue-specific, temporal changes in cell adhesion to echinonectin in the sea urchin embryo". Developmental Biology. 144 (2): 327–34. doi:10.1016/0012-1606(91)90425-3. PMID 1707016.
- ↑ Miller, JR; McClay, DR (1997). "Characterization of the Role of Cadherin in Regulating Cell Adhesion during Sea Urchin Development". Developmental Biology. 192 (2): 323–39. doi:10.1006/dbio.1997.8740. PMID 9441671.
- ↑ Miller, JR; McClay, DR (1997). "Changes in the pattern of adherens junction-associated beta-catenin accompany morphogenesis in the sea urchin embryo". Developmental Biology. 192 (2): 310–22. doi:10.1006/dbio.1997.8739. PMID 9441670.
- ↑ Anstrom, JA (1989). "Sea urchin primary mesenchyme cells: ingression occurs independent of microtubules". Developmental Biology. 131 (1): 269–75. doi:10.1016/S0012-1606(89)80058-2. PMID 2562830.
- ↑ Anstrom, JA (1992). "Microfilaments, cell shape changes, and the formation of primary mesenchyme in sea urchin embryos". The Journal of experimental zoology. 264 (3): 312–22. doi:10.1002/jez.1402640310. PMID 1358997.
- ↑ Kazuya Yoshimura, Tomoaki Iketani et Tatsuo Motokawa, "Do regular sea urchins show preference in which part of the body they orient forward in their walk ?", Marine Biology, vol. 159, no 5, 2012, p. 959-965.
- ↑ "Defence – spines". Echinoid Directory. Natural History Museum.
- ↑ "Defence – pedicellariae". Echinoid Directory. Natural History Museum.
- ↑ Hiroko Sakashita, " Sexual dimorphism and food habits of the clingfish, Diademichthys lineatus, and its dependence on host sea urchin ", Environmental Biology of Fishes, vol. 34, no 1, 1994, p. 95-101
- ↑ Baumiller, Tomasz K. (2008). "Crinoid Ecological Morphology". Annual Review of Earth and Planetary Sciences. 36: 221–49. Bibcode:2008AREPS..36..221B. doi:10.1146/annurev.earth.36.031207.124116.
- ↑ Mattison, JE; Trent, JD; Shanks, AL; Akin, TB; Pearse, JS (1977). "Movement and feeding activity of red sea urchins (Strongylocentrotus franciscanus) adjacent to a kelp forest". Marine Biology. 39 (1): 25–30. doi:10.1007/BF00395589.
- 1 2 3 Konar, Brenda (2000). "Habitat influences on sea urchin populations". In: Hallock and French (eds). Diving for Science...2000. Proceedings of the 20th Annual Scientific Diving Symposium. American Academy of Underwater Sciences. Retrieved 2011-01-07.
- ↑ "Aquatic Species at Risk – Species Profile – Sea Otter". Fisheries and Oceans Canada. Archived from the original on 2008-01-23. Retrieved 2007-11-29.
- 1 2 3 Kroh, Andreas (2010). "The phylogeny and classification of post-Palaeozoic echinoids". Journal of Systematic Palaeontology. 8 (2): 147–212. doi:10.1080/14772011003603556..
- ↑ Mah, Christopher (April 12, 2011). "Sizes and Species in the Strangest of the Strange : Deep-Sea Pourtalesiid Urchins". The Echinoblog..
- 1 2 Mah, Christopher (April 8, 2014). "What are the Deepest known echinoderms ?". The Echinoblog..
- ↑ ChrisM. "The Echinoblog". echinoblog.blogspot.com.
- ↑ Pereira, Bruno; Benton, M; Ruta, M; Mateus, O (2015). "Mesozoic echinoid diversity in Portugal: Investigating fossil record quality and environmental constraints on a regional scale.". Palaeogeography, Palaeoclimatology, Palaeoecology. 424: 132–146. doi:10.1016/j.palaeo.2015.02.014.
- ↑ Davidson, Eric (2002). "PROPOSAL FOR SEQUENCING FURTHER ECHINODERM GENOMES FOR THE PURPOSES OF GLOBAL GENE REGULATORY NETWORK ANALYSIS" (PDF).
- ↑ Kroh, A.; Hansson, H. (2013). "Echinoidea (Leske, 1778)". World Register of Marine Species. Retrieved 2014-01-04.
- ↑ "Sea Urchin Puncture Wound: Get Facts About Treatment". eMedicineHealth.
- ↑ "Insight from the Sea Urchin". Microscope Imaging Station. Exploratorium. Retrieved 2013-02-04.
- ↑ "The Genome of the Sea Urchin Strongylocentrotus purpuratus". Science (New York, N.Y.). 314 (5801): 941–952. 2006-11-10. doi:10.1126/science.1133609. ISSN 0036-8075. PMC 3159423
 . PMID 17095691.
. PMID 17095691. - ↑ "California Purple Sea-Urchin Genome Sequenced by International Team | Caltech". The California Institute of Technology. Retrieved 2016-12-05.
- ↑ "Sea Urchin Genome Project". sugp.caltech.edu. Retrieved 2016-12-05.
- ↑ "The Genome of the Sea Urchin Strongylocentrotus purpuratus". Science (New York, N.Y.). 314 (5801): 941–952. 2006-11-10. doi:10.1126/science.1133609. ISSN 0036-8075. PMC 3159423
 . PMID 17095691.
. PMID 17095691. - ↑ Rast, JP; Smith, LC; Loza-Coll, M; Hibino, T; Litman, GW (2006). "Genomic Insights into the Immune System of the Sea Urchin". Science. 314 (5801): 952–6. Bibcode:2006Sci...314..952R. doi:10.1126/science.1134301. PMID 17095692.
- ↑ "The Genome of the Sea Urchin Strongylocentrotus purpuratus". Science (New York, N.Y.). 314 (5801): 941–952. 2006-11-10. doi:10.1126/science.1133609. ISSN 0036-8075. PMC 3159423
 . PMID 17095691.
. PMID 17095691. - ↑ Bodnar, Andrea G.; Coffman, James A. (2016-08-01). "Maintenance of somatic tissue regeneration with age in short- and long-lived species of sea urchins". Aging Cell. 15 (4): 778–787. doi:10.1111/acel.12487. ISSN 1474-9726. PMC 4933669
 . PMID 27095483.
. PMID 27095483. - ↑ "2012 | Sea urchins could contain the genetic key to curing some diseases | University of St Andrews". www.st-andrews.ac.uk. Retrieved 2016-12-05.
- ↑ "Stanford seeks sea urchin's secret to surviving ocean acidification | Stanford News Release". news.stanford.edu. Retrieved 2016-12-05.
- ↑ Laura Rogers-Bennett, "The Ecology of Strongylocentrotus franciscanus and Strongylocentrotus purpuratus" in John M. Lawrence, Edible sea urchins: biology and ecology, p. 410
- ↑ Alan Davidson, Oxford Companion to Food, s.v. sea urchin
- ↑ John M. Lawrence, "Sea Urchin Roe Cuisine" in John M. Lawrence, Edible sea urchins: biology and ecology
- ↑ for Puglia, Italy: Touring Club Italiano, Guida all'Italia gastronomica, 1984, p. 314; for Alexandria, Egypt: Claudia Roden, A Book of Middle Eastern Food, p. 183
- ↑ Alan Davidson, Mediterranean Seafood, p. 270
- ↑ Larousse Gastronomique
- ↑ Curnonsky, Cuisine et vins de France, nouvelle édition, 1974, p. 248
- ↑ Davidson, p. 280
- ↑ Kleiman, Dena (October 3, 1990). "Scorned at Home, Maine Sea Urchin Is a Star in Japan". New York Times. p. C1.
- ↑ Davidson, Oxford Companion
- ↑ Wassilieff, Maggy (March 2, 2009). "sea urchins". Te Ara Encyclopedia of New Zealand.
- ↑ Macey, Richard (November 9, 2004). "The little urchins that can command a princely price". The Sydney Morning Herald.
- ↑ "Sea Urchin Fishery and Overfishing", TED Case Studies 296, American University full text
- ↑ Martin, R.E.; Carter, E.P.; Flick, G.J.; Davis, L.M. (2000). Marine and Freshwater Products Handbook. Taylor & Francis. p. 268. ISBN 978-1-56676-889-4. Retrieved 2014-12-03.
External links
| Wikimedia Commons has media related to Sea urchin. |
| Wikispecies has information related to: Echinoidea |
| Wikisource has the text of the 1905 New International Encyclopedia article Sea-Urchin. |
- "Sea Urchins Factsheet". Waitt Institute. Retrieved 2015-06-08.
- World Register of Marine Species link: Echinoidea Leske, 1778 (+species list)
- Plankton Chronicles Short documentary films & photos
- The sea urchin genome project
- Sea Urchin Harvesters Association – California Also, (604) 524-0322.
- The Echinoid Directory from the Natural History Museum.
- Echinoids of the North Sea
- Spiny creature's genome insight
- Echinoids.nl
- lantern.jpg A labeled diagram of the sea urchin's Aristotle's lantern.
- aristotle.htm Who is this person Aristotle and what about this lantern?
- www.emilydamstra.com Illustration of the musculature of an Aristotle's lantern.
- Urchin Anatomy a flash about the anatomy of the sea urchin
- www.sea-urchins.com An article about sea-urchin parasites.
- Further research on sea urchins
- Photographic Database of Cambodian Sea Urchins
- California Sea Urchin commission
- Introduction to the Echinoidea
- 70% of Sea Urchin Genes Have a Human Counterpart—Sequencing confirms that sea urchins are more closely related to humans than fruit flies (LiveScience.com, November 2006).






.jpg)

.jpg)




.png)
