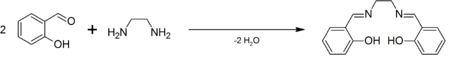Salen ligand
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
2,2′-Ethylenebis(nitrilomethylidene)diphenol, N,N′-Ethylenebis(salicylimine) | |
| Identifiers | |
| 94-93-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL594100 |
| ChemSpider | 10484366 |
| ECHA InfoCard | 100.002.161 |
| UNII | M122L9EGR6 |
| |
| |
| Properties | |
| C16H16N2O2 | |
| Molar mass | 268.31 |
| Appearance | yellow solid |
| Melting point | 125 to 129 °C (257 to 264 °F; 398 to 402 K) |
| organic solvents | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Salen is the abbreviation for a popular chelating ligand used in coordination chemistry and homogeneous catalysis. The name salen is a contraction for salicylaldehyde and ethylenediamine. The ligand is a bright yellow micaceous solid that is soluble in polar organic solvents.
Nomenclature
The diphenol H2salen is the conjugate acid of the ligand that logically is salen2−. But the terminology is used loosely. As an anionic tetradendate ligand, salen2− resembles tetradentate ligands including those that are macrocyclic, such as porphyrinate, corrin, bis(dimethylglyoximate), and some Schiff bases.
Preparation
H2salen is commercially available. It was first prepared by Pfeiffer.[1] It is often generated in situ followed by the addition of the metal salt, but the ligand is also easily prepared as a pure organic compound by the condensation of ethylenediamine and salicylaldehyde.[2]
Acacen ligands
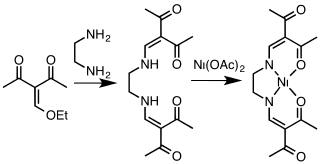
Related to salen ligands but based on non-aromatic precursors are the acacen ligands. These dianionic tetradentate ligands are often derived from the condensation of ketoaldehyde equivalents and diamines. The name acacen derives from the abbreviation for acetylacetone and ethylenediamine.[3][4]
References
- ↑ Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. (1933). "Tricyclische orthokondensierte Nebenvalenzringe" [Tricyclic ortho-condensed outer valence rings]. Liebigs Ann. Chem. 503: 84–130. doi:10.1002/jlac.19335030106.
- ↑ Diehl, Harvey; Hach, Clifford C. (1950). "Bis(N,N′-Disalicylalethylenediamine)-μ-Aquodicobalt(II)". Inorg. Synth. 3: 196–201. doi:10.1002/9780470132340.ch53. ISBN 978-0-470-13234-0.
- 1 2 Weber, Birgit; Jäger, Ernst-G. (2009). "Structure and Magnetic Properties of Iron(II/III) Complexes with N
2O2–
2-Coordinating Schiff Base-Like Ligands". Eur. J. Inorg. Chem.: 455. doi:10.1002/ejic.200990003. - ↑ Riley, Dennis P.; Busch, Daryle H. (1978). "Macrocyclic Tetraazatetraenato Ligands and their Metal Complexes". Inorg. Synth. 18: 36. doi:10.1002/9780470132494.ch7.
See also
- Metal salen complexes
- Bisthiosemicarbazones - a structurally related class of C2-symmetric imine based ligands