S-layer
An S-layer (surface layer) is a part of the cell envelope commonly found in archaea, as well as bacteria.[1][2] It consists of a monomolecular layer composed of identical proteins or glycoproteins. This structure is built via self-assembly and encloses the whole cell surface. Thus, the S-layer protein can represent up to 10–15% of the whole protein content of a cell.[3][4][5] S-layer proteins are poorly conserved or not conserved at all, and can differ markedly even between related species. Depending on species, the S-layers have a thickness between 5 and 25 nm and possess identical pores with 2–8 nm in diameter.[6] S-layers exhibit either an oblique (p1, p2), square (p4) or hexagonal (p3, p6) lattice symmetry. Depending on the lattice symmetry, the S-layer is composed of one (P1), two (P2), three (P3), four (P4), or six (P6) identical protein subunits, respectively. The center-to-center spacings (or unit cell dimensions) between these subunits range between 2.5 and 35 nm.
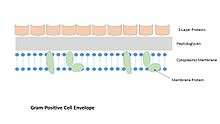
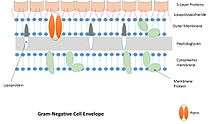
Fixation of S-layers in the cell wall
- In Gram-negative bacteria, S-layers are associated to the lipopolysaccharides via ionic, carbohydrate–carbohydrate, protein–carbohydrate interactions and/or protein–protein interactions.
- In Gram-positive bacteria whose S-layers often contain surface layer homology (SLH) domains, the binding occurs to the peptidoglycan and to a secondary cell wall polymer (e.g., teichoic acids). In the absence of SLH domains, the binding occurs via electrostatic interactions between the positively charged N-terminus of the S-layer protein and a negatively charged secondary cell wall polymer.
- In Gram-negative archaea, S-layer proteins possess a hydrophobic anchor that is associated with the underlying lipid membrane.
- In Gram-positive archaea, the S-layer proteins bind to pseudomurein or to methanochondroitin.
Biological functions of the S-layer[7] For many bacteria, the S-layer represents the outermost interaction zone with their respective environment. Its functions are very diverse and vary from species to species. In many archaeal species the S-layer is the only cell wall component and, therefore, is important for mechanical and osmotic stabilization. Additional functions associated with S-layers include:
- protection against bacteriophages, Bdellovibrios, and phagocytosis
- resistance against low pH
- barrier against high-molecular-weight substances (e.g., lytic enzymes)
- adhesion (for glycosylated S-layers)
- stabilisation of the membrane
- provision of adhesion sites for exoproteins
- provision of a periplasmic compartment in Gram-positive prokaryotes together with the peptidoglycan and the cytoplasmic membranes
S-layer structure
While ubiquitous among Archaea, and common in bacteria, the S-layers of diverse organisms have unique structural properties, including symmetry and unit cell dimensions, due to fundamental differences in their constituent building blocks. Sequence analyses of S-layer proteins have predicted that S-layer proteins have sizes of 40-200 kDa and may be composed of multiple domains some of which may be structurally related. Since their discovery in the 1950s[8] S-layer structure has been investigated extensively by electron microscopy and medium resolution images of S-layers from these analyses has provided useful information on overall S-layer morphology. High-resolution structures of an archaeal S-layer protein (MA0829 from Methanosarcina acetivorans C2A) of the Methanosarcinales S-layer Tile Protein family and a bacterial S-layer protein (SbsB), from Geobacillus stearothermophilus PV72, have recently been determined by X-ray crystallography.[9][10] In contrast with existing crystal structures, which have represented individual domains of S-layer proteins or minor proteinaceous components of the S-layer, the MA0829 and SbsB structures have allowed high resolution models of the M. acetivorans and G. stearothermophilus S-layers to be proposed. These models exhibit hexagonal (p6) and oblique (p2) symmetry, for M. acetivorans and G. stearothermophilus S-layers, respectively, and their molecular features, including dimensions and porosity, are in good agreement with data from electron microscopy studies of archaeal and bacterial S-layers.
References
- ↑ Sára, Margit; Sleytr, Uwe B. (2000-02-15). "S-Layer Proteins". Journal of Bacteriology. 182 (4): 859–868. doi:10.1128/JB.182.4.859-868.2000. ISSN 0021-9193. PMC 94357
 . PMID 10648507.
. PMID 10648507. - ↑ S-layers on cell walls of cyanobacteria Micron Volume 33, Issue 3, January 2002, Pages 257–277; doi:10.1016/S0968-4328(01)00031-2
- ↑ Messner P, Sleytr U (1992). "Crystalline bacterial cell-surface layers". Adv. Microb. Physiol. 33: 213–75. doi:10.1016/S0065-2911(08)60218-0. PMID 1636510.
- ↑ Pum D, Messner P, Sleytr U (1991). "Role of the S layer in morphogenesis and cell division of the archaebacterium Methanocorpusculum sinense". J. Bacteriol. 173 (21): 6865–73. PMC 209039
 . PMID 1938891.
. PMID 1938891. - ↑ Sleytr U, Messner P, Pum D, Sára M (1993). "Crystalline bacterial cell surface layers". Mol. Microbiol. 10 (5): 911–6. doi:10.1111/j.1365-2958.1993.tb00962.x. PMID 7934867.
- ↑ Sleytr U, Bayley H, Sára M, Breitwieser A, Küpcü S, Mader C, Weigert S, Unger F, Messner P, Jahn-Schmid B, Schuster B, Pum D, Douglas K, Clark N, Moore J, Winningham T, Levy S, Frithsen I, Pankovc J, Beale P, Gillis H, Choutov D, Martin K (1997). "Applications of S-layers". FEMS Microbiol. Rev. 20 (1–2): 151–75. doi:10.1016/S0168-6445(97)00044-2. PMID 9276930.
- ↑ Sleytr, UB; Beveridge, TJ (1999). "Bacterial S-layers". Trends Microbiol. 7 (6): 253-260. PMID 10366863.
- ↑ Houwink, AL (1953). "A macromolecular mono-layer in the cell wall of Spirillum spec.". Biochim Biophys Acta. 10 (3): 360–6. doi:10.1016/0006-3002(53)90266-2. PMID 13058992.
- ↑ Arbing MA, Chan S, Shin A, Phan T, Ahn CJ, Rohlin L, Gunsalus RP (2012). "Structure of the surface layer of the methanogenic archaean Methanosarcina acetivorans.". Proc Natl Acad Sci U S A. 109 (29): 11812–7. doi:10.1073/pnas.1120595109. PMC 3406845
 . PMID 22753492.
. PMID 22753492. - ↑ Baranova E, Fronzes R, Garcia-Pino A, Van Gerven N, Papapostolou D, Péhau-Arnaudet G, Pardon E, Steyaert J, Howorka S, Remaut H (2012). "SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly". Nature. 487 (7405): 119–22. doi:10.1038/nature11155. PMID 22722836.