Riboflavin
 | |
| Names | |
|---|---|
| IUPAC name
7,8-Dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4-dione[1] | |
| Identifiers | |
| 83-88-5 | |
| 3D model (Jmol) | Interactive image |
| 3DMet | B01201 |
| 97825 | |
| ChEBI | CHEBI:17015 |
| ChEMBL | ChEMBL1534 |
| ChemSpider | 431981 |
| DrugBank | DB00140 |
| ECHA InfoCard | 100.001.370 |
| EC Number | 201-507-1 |
| E number | E101 (colours) |
| 6578 | |
| KEGG | D00050 |
| MeSH | Riboflavin |
| PubChem | 493570 |
| UNII | TLM2976OFR |
| |
| |
| Properties | |
| C17H20N4O6 | |
| Molar mass | 376.37 g·mol−1 |
| Appearance | Orange crystals |
| log P | 0.095 |
| Acidity (pKa) | 9.888 |
| Basicity (pKb) | 4.109 |
| Pharmacology | |
| A11HA04 (WHO) | |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

Riboflavin (vitamin B2) is part of the vitamin B group. It is the central component of the cofactors FAD and FMN and as such required for a variety of flavoprotein enzyme reactions including activation of other vitamins. It was formerly known as vitamin G.[2]
As a chemical compound, riboflavin is a yellow-orange solid substance with poor solubility in water compared to other B vitamins. Visually, it imparts color to vitamin supplements (and bright yellow color to the urine of persons taking a lot of it).
The name "riboflavin" (often abbreviated to Rbf or RBF)[3][4] comes from "ribose" (the sugar whose reduced form, ribitol, forms part of its structure) and "flavin", the ring-moiety which imparts the yellow color to the oxidized molecule (from Latin flavus, "yellow"). The reduced form, which occurs in metabolism along with the oxidized form, is colorless.
Function
Riboflavin functions as a coenzyme, meaning that it is required for enzymes (proteins) to perform normal physiological actions. Specifically, the active forms of riboflavin flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) function as cofactors for a variety of flavoproteine enzyme reactions:
- Flavoproteins of electron transport chain, including FMN in Complex I and FAD in Complex II
- FAD is required for the production of pyridoxic acid from pyridoxal (vitamin B6) by pyridoxine 5'-phosphate oxidase
- The primary coenzyme form of vitamin B6 (pyridoxal phosphate) is FMN dependent
- Oxidation of pyruvate, α-ketoglutarate, and branched-chain amino acids requires FAD in the shared E3 portion of their respective dehydrogenase complexes
- Fatty acyl CoA dehydrogenase requires FAD in fatty acid oxidation
- FAD is required to convert retinol (vitamin A) to retinoic acid via cytosolic retinal dehydrogenase
- Synthesis of an active form of folate (5-methyltetrahydrofolate) from 5,10-methylenetetrahydrofolate by Methylenetetrahydrofolate reductase is FADH2 dependent
- FAD is required to convert tryptophan to niacin (vitamin B3)
- Reduction of the oxidized form of glutathione (GSSG) to its reduced form (GSH) by Glutathione reductase is FAD dependent
For the molecular mechanism of action see main articles Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD)
Food sources
Food and beverages that provide riboflavin without fortification are milk, cheese, eggs, leaf vegetables, liver, kidneys, legumes, mushrooms, and almonds.[5]
The milling of cereals results in considerable loss (up to 60%) of vitamin B2, so white flour is enriched in some countries such as US by addition of the vitamin. The enrichment of bread and ready-to-eat breakfast cereals contributes significantly to the dietary supply of vitamin B2. Polished rice is not usually enriched, because the vitamin’s yellow color would make the rice visually unacceptable to the major rice-consumption populations. However, most of the flavin content of whole brown rice is retained if the rice is steamed (parboiled) prior to milling. This process drives the flavins in the germ and aleurone layers into the endosperm. Free riboflavin is naturally present in foods along with protein-bound FMN and FAD. Bovine milk contains mainly free riboflavin, with a minor contribution from FMN and FAD. In whole milk, 14% of the flavins are bound noncovalently to specific proteins.[6] Egg white and egg yolk contain specialized riboflavin-binding proteins, which are required for storage of free riboflavin in the egg for use by the developing embryo.
Riboflavin is added to baby foods, breakfast cereals, pastas and vitamin-enriched meal replacement products. It is difficult to incorporate riboflavin into liquid products because it has poor solubility in water, hence the requirement for riboflavin-5'-phosphate (E101a), a more soluble form of riboflavin. Riboflavin is also used as a food coloring and as such is designated in Europe as the E number E101.[7]
Dietary reference intake
The Food and Nutrition Board of the U.S. Institute of Medicine updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) in 1998. The current EARs for riboflavin for women and men ages 14 and up are 0.9 mg/day and 1.1 mg/day, respectively; the RDAs are 1.1 and 1.3 mg/day. RDAs are higher than EARs so as to identify amounts that will cover people with higher than average requirements. RDA for pregnancy equals 1.4 mg/day. RDA for lactation equals 1.6 mg/day. For infants up to 12 months the Adequate Intake (AI) is 0.3-0.4 mg/day and for children ages 1–13 years the RDA increases with age from 0.5 to 0.9 mg/day. Collectively the EARs, RDAs and ULs (see Toxicity) are referred to as Dietary Reference Intakes.[8][9]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For riboflavin labeling purposes 100% of the Daily Value was 1.7 mg, but as of May 2016 it has been revised to 1.3 mg. A table of the pre-change adult Daily Values is provided at Reference Daily Intake. Food and supplement companies have until July 2018 to comply with the change.
Deficiency
Signs and symptoms
In humans
Mild deficiencies can exceed 50% of the population in third world countries and in refugee situations. Deficiency is uncommon in the United States and in other countries that have wheat flour, bread, pasta, corn meal or rice enrichment regulations. In the U.S., starting in the 1940s, flour, corn meal and rice have been fortified with B vitamins as a means of restoring some of what is lost in milling, bleaching and other processing. For adults 20 and older, average intake from food and beverages is 1.8 mg/day for women and 2.5 mg/day for men. An estimated 23% consume a riboflavin-containing dietary supplement that provides on average 10 mg. The U.S. Department of Health and Human Services conducts National Health and Nutrition Examination Survey every two years and reports food results in a series of reports referred to as "What We Eat In America." From NHANES 2011–2012, the latest for which data has been reported, estimates are that 8% of women and 3% of men consume less than the RDA. When compared to the lower Estimated Average Requirements, fewer than 3% do not achieve the EAR level. However, anyone choosing a gluten-free or low gluten diet should as a precaution take a multi-vitamin/mineral dietary supplement which provides 100% DV for riboflavin and other B vitamins.
Riboflavin deficiency (also called ariboflavinosis) results in stomatitis including painful red tongue with sore throat, chapped and fissured lips (cheilosis), and inflammation of the corners of the mouth (angular stomatitis). There can be oily scaly skin rashes on the scrotum, vulva, philtrum of the lip, or the nasolabial folds. The eyes can become itchy, watery, bloodshot and sensitive to light.[10] Due to interference with iron absorption, even mild to moderate riboflavin deficiency results in an anemia with normal cell size and normal hemoglobin content (i.e. normochromic normocytic anemia). This is distinct from anemia caused by deficiency of folic acid (B9) or cyanocobalamin (B12), which causes anemia with large blood cells (megaloblastic anemia).[11] Deficiency of riboflavin during pregnancy can result in birth defects including congenital heart defects[12] and limb deformities.[13]
The stomatitis symptoms are similar to those seen in pellagra, which is caused by niacin (B3) deficiency. Therefore, riboflavin deficiency is sometimes called "pellagra sine pellagra" (pellagra without pellagra), because it causes stomatitis but not widespread peripheral skin lesions characteristic of niacin deficiency.[10]
Riboflavin has been noted to prolong recovery from malaria,[14] despite preventing growth of plasmodium (the malaria parasite).[15]
In other animals
In other animals, riboflavin deficiency results in lack of growth,[16] failure to thrive, and eventual death. Experimental riboflavin deficiency in dogs results in growth failure, weakness, ataxia, and inability to stand. The animals collapse, become comatose, and die. During the deficiency state, dermatitis develops together with hair loss. Other signs include corneal opacity, lenticular cataracts, hemorrhagic adrenals, fatty degeneration of the kidney and liver, and inflammation of the mucous membrane of the gastrointestinal tract.[17] Post-mortem studies in rhesus monkeys fed a riboflavin-deficient diet revealed about one-third the normal amount of riboflavin was present in the liver, which is the main storage organ for riboflavin in mammals.[18] Riboflavin deficiency in birds results in low egg hatch rates.[19]
Diagnosis
Overt clinical signs are rarely seen among inhabitants of the developed countries. The assessment of Riboflavin status is essential for confirming cases with unspecific symptoms where deficiency is suspected.
- Glutathione reductase is a nicotinamide adenine dinucleotide phosphate (NADPH) and FAD-dependent enzyme, and the major flavoprotein in erythrocyte. The measurement of the activity coefficient of erythrocyte glutathione reductase (EGR) is the preferred method for assessing riboflavin status.[20] It provides a measure of tissue saturation and long-term riboflavin status. In vitro enzyme activity in terms of activity coefficients (AC) is determined both with and without the addition of FAD to the medium. ACs represent a ratio of the enzyme’s activity with FAD to the enzyme’s activity without FAD. An AC of 1.2 to 1.4, riboflavin status is considered low when FAD is added to stimulate enzyme activity. An AC > 1.4 suggests riboflavin deficiency. On the other hand, if FAD is added and AC is < 1.2, then riboflavin status is considered acceptable.[9] Tillotson and Bashor[21] reported that a decrease in the intakes of riboflavin was associated with increase in EGR AC. In the UK study of Norwich elderly,[22] initial EGR AC values for both males and females were significantly correlated with those measured 2 years later, suggesting that EGR AC may be a reliable measure of long-term biochemical riboflavin status of individuals. These findings are consistent with earlier studies.[23]
- Experimental balance studies indicate that urinary riboflavin excretion rates increase slowly with increasing intakes, until intake level approach 1.0 mg/d, when tissue saturation occurs. At higher intakes, the rate of excretion increases dramatically.[24] Once intakes of 2.5 mg/d are reached, excretion becomes approximately equal to the rate of absorption (Horwitt et al., 1950) (18). At such high intake a significant proportion of the riboflavin intake is not absorbed. If urinary riboflavin excretion is <19 µg/g creatinine (without recent riboflavin intake) or < 40 µg per day are indicative of deficiency.
Causes
Riboflavin is continuously excreted in the urine of healthy individuals,[25] making deficiency relatively common when dietary intake is insufficient.[25] Riboflavin deficiency is usually found together with other nutrient deficiencies, particularly of other water-soluble vitamins. A deficiency of riboflavin can be primary - poor vitamin sources in one's daily diet - or secondary, which may be a result of conditions that affect absorption in the intestine, the body not being able to use the vitamin, or an increase in the excretion of the vitamin from the body. Subclinical deficiency has also been observed in women taking oral contraceptives, in the elderly, in people with eating disorders, chronic alcoholism and in diseases such as HIV, inflammatory bowel disease, diabetes and chronic heart disease. The Celiac Disease Foundation points out that a gluten-free diet may be low in riboflavin (and other nutrients) as enriched wheat flour and wheat foods (bread, pasta, cereals, etc.) is a major dietary contribution to total riboflavin intake. Phototherapy to treat jaundice in infants can cause increased degradation of riboflavin, leading to deficiency if not monitored closely.
Treatment
Treatment involves a diet which includes an adequate amount of riboflavin containing foods. Multi-vitamin and mineral dietary supplements often contain 100% of the Daily Value for riboflavin, and can be used by persons concerned about an inadequate diet. Over-the-counter dietary supplements are available in the United States with doses as high as 100 mg (5882% of Daily Value), but there is no evidence that these high doses have any additional benefit for healthy people.
Medical uses
Riboflavin has been used in several clinical and therapeutic situations. For over 30 years, riboflavin supplements have been used as part of the phototherapy treatment of neonatal jaundice. The light used to irradiate the infants breaks down not only bilirubin, the toxin causing the jaundice, but also the naturally occurring riboflavin within the infant's blood, so extra supplementation is necessary.
One clinical trial found that high-dose riboflavin appears to be useful alone or along with beta-blockers in the prevention of migraine.[26][27] A dose of 400 mg daily has been used effectively in the prophylaxis of migraines, especially in combination with a daily supplement of magnesium citrate 500 mg and, in some cases, a supplement of coenzyme Q10.[28] However, two other clinical studies have failed to find any significant results for the effectiveness of B2 as a treatment for migraine.[29][30]
Riboflavin in combination with UV light has been shown to be effective in reducing the ability of harmful pathogens found in blood products to cause disease.[31][32][33] When UV light is applied to blood products containing riboflavin, the nucleic acids in pathogens are damaged, rendering them unable to replicate and cause disease.[33][34] Riboflavin and UV light treatment has been shown to be effective for inactivating pathogens in platelets and plasma, and is under development for application to whole blood. Because platelets and red blood cells do not contain a nucleus (i.e. they have no DNA to be damaged) the technique is well-suited for destroying nucleic acid containing pathogens (including viruses, bacteria, parasites, and white blood cells) in blood products.[35]
Corneal ectasia is a progressive thinning of the cornea; the most common form of this condition is keratoconus. Collagen cross-linking is a non-surgical treatment intended to slow progression of corneal ectasia by strengthening corneal tissue. The standard protocol calls for application directly to the eye of a 0.1% riboflavin solution for 30 minutes followed by 30 minutes of ultraviolet-A irradiation with a wavelength of 370 nm and power of 3 mW/cm2.[36]
Industrial uses
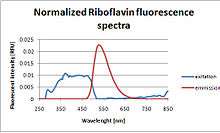

Because riboflavin is fluorescent under UV light, dilute solutions (0.015-0.025% w/w) are often used to detect leaks or to demonstrate coverage in an industrial system such a chemical blend tank or bioreactor. (See the ASME BPE section on Testing and Inspection for additional details.)
Toxicity
In humans, there is no evidence for riboflavin toxicity produced by excessive intakes, in part because it has lower water solubility than other B vitamins, because absorption becomes less efficient as doses increase, and because what excess is absorbed is excreted via the kidneys into urine. Even when 400 mg of riboflavin per day was given orally to subjects in one study for three months to investigate the efficacy of riboflavin in the prevention of migraine headache, no short-term side effects were reported.[9][37][38] Although toxic doses can be administered by injection,[37] any excess at nutritionally relevant doses is excreted in the urine,[39] imparting a bright yellow color when in large quantities.
The Food and Nutrition Board of the U.S. Institute of Medicine sets Tolerable Upper Intake Levels (known as ULs) for vitamins and minerals when evidence is sufficient. In the case of riboflavin there is no UL, as there is no human data for adverse effects from high doses. The European Food Safety Authority reviewed the same safety question and also reached the conclusion that there was not sufficient evidence to set a UL for riboflavin.[40]
Industrial synthesis

Various biotechnological processes have been developed for industrial scale riboflavin biosynthesis using different microorganisms, including filamentous fungi such as Ashbya gossypii, Candida famata and Candida flaveri, as well as the bacteria Corynebacterium ammoniagenes and Bacillus subtilis.[41] The latter organism has been genetically modified to both increase the bacteria's production of riboflavin and to introduce an antibiotic (ampicillin) resistance marker, and is now successfully employed at a commercial scale to produce riboflavin for feed and food fortification purposes. The chemical company BASF has installed a plant in South Korea, which is specialized on riboflavin production using Ashbya gossypii. The concentrations of riboflavin in their modified strain are so high, that the mycelium has a reddish/brownish color and accumulates riboflavin crystals in the vacuoles, which will eventually burst the mycelium. Riboflavin is sometimes overproduced, possibly as a protective mechanism, by certain bacteria in the presence of high concentrations of hydrocarbons or aromatic compounds. One such organism is Micrococcus luteus (American Type Culture Collection strain number ATCC 49442), which develops a yellow color due to production of riboflavin while growing on pyridine, but not when grown on other substrates, such as succinic acid.[42]
History
Vitamin B was originally considered to have two components, a heat-labile vitamin B1 and a heat-stable vitamin B2. In the 1920s, vitamin B2 was thought to be the factor necessary for preventing pellagra. In 1923, Paul Gyorgy in Heidelberg was investigating egg-white injury in rats; the curative factor for this condition was called vitamin H (which is now called biotin or vitamin B7). Since both pellagra and vitamin H deficiency were associated with dermatitis, Gyorgy decided to test the effect of vitamin B2 on vitamin H deficiency in rats. He enlisted the service of Wagner-Jauregg in Kuhn’s laboratory. In 1933, Kuhn, Gyorgy, and Wagner found that thiamin-free extracts of yeast, liver, or rice bran prevented the growth failure of rats fed a thiamin-supplemented diet.
Further, the researchers noted that a yellow-green fluorescence in each extract promoted rat growth, and that the intensity of fluorescence was proportional to the effect on growth. This observation enabled them to develop a rapid chemical and bioassay to isolate the factor from egg white in 1933, they called it Ovoflavin. The same group then isolated the same preparation (a growth-promoting compound with yellow-green fluorescence) from whey using the same procedure (lactoflavin). In 1934 Kuhn’s group identified the structure of so-called flavin and synthesized vitamin B2.
See also
References
- ↑ CID 493570 from PubChem
- ↑ David Bennett (27 July 2013). "Every Vitamin Page: All Vitamins and Pseudo-Vitamins" (PDF). lifeinyouryears.net. Retrieved 17 May 2014.
- ↑ Handbook of Behavior, Food and Nutrition, edited by Victor R. Preedy, Ronald Ross Watson, Colin R. Martin, Springer Science & Business Media, 15 Apr 2011, Ch.153, p.2428, ISBN 9780387922713
- ↑ Redox reactions of reduced flavin mononucleotide (FMN), riboflavin (RBF), and anthraquinone-2,6-disulfonate (AQDS) with ferrihydrite and lepidocrocite, Shi Z, Zachara JM, Shi L, Wang Z, Moore DA, Kennedy DW, Fredrickson JK, Environ Sci Technol. 2012 Nov 6;46(21):11644-52. doi: 10.1021/es301544b. Epub 2012 Oct 24
- ↑ Higdon, Jane; Victoria J. Drake (2007). "Riboflavin". Micronutrient Information Center. Linus Pauling Institute at Oregon State University. Retrieved December 3, 2009.
- ↑ Kanno, C., Kanehara, N., Shirafuji, K., and et al. Binding Form of Vitamin B2 in Bovine Milk: its concentration, distribution, and binding linkage, J. Nutr. Sci. Vitaminol., 37, 15, 1991
- ↑ "Current EU approved additives and their E Numbers". UK Food Standards Agency. July 27, 2007. Retrieved December 3, 2009.
- ↑ Riboflavin. IN: Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press. 1998, PP.87-122.
- 1 2 3 Gropper, SS; Smith, JL; Groff, JL (2009). "Ch. 9: Riboflavin". Advanced Nutrition and Human Metabolism (5th ed.). Wadsworth: CENGAG Learning. pp. 329–33.
- 1 2 Sebrell, W. H.; R. E. Butler (1939). "Riboflavin Deficiency in Man (Ariboflavinosis)". Public Health Reports. 54 (48): 2121–2131. doi:10.2307/4583104. ISSN 0094-6214. JSTOR 4583104.
- ↑ Lane M, Alfrey CP (Apr 1965). "THE ANEMIA OF HUMAN RIBOFLAVIN DEFICIENCY". Blood. 25: 432–442. PMID 14284333.
- ↑ Smedts HP, Rakhshandehroo M, Verkleij-Hagoort AC, de Vries JH, Ottenkamp J, Steegers EA, Steegers-Theunissen RP (Oct 2008). "Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects". European Journal of Nutrition. 47 (7): 357–365. doi:10.1007/s00394-008-0735-6. PMID 18779918.
- ↑ Robitaille J, Carmichael SL, Shaw GM, Olney RS (Sep 2009). "Maternal nutrient intake and risks for transverse and longitudinal limb deficiencies: data from the National Birth Defects Prevention Study, 1997–2003". Birth Defects Research. Part A, Clinical and Molecular Teratology. 85 (9): 773–779. doi:10.1002/bdra.20587. PMID 19350655.
- ↑ Das BS, Das DB, Satpathy RN, Patnaik JK, Bose TK (Apr 1988). "Riboflavin deficiency and severity of malaria". European Journal of Clinical Nutrition. 42 (4): 277–283. PMID 3293996.
- ↑ Dutta, Purabi; John Pinto; Richard Rivlin (1985). "ANTIMALARIAL EFFECTS OF RIBOFLAVIN DEFICIENCY". The Lancet. Originally published as Volume 2, Issue 8463. 326 (8463): 1040–1043. doi:10.1016/S0140-6736(85)90909-2. ISSN 0140-6736. Retrieved 2015-02-14.
- ↑ Patterson BE, Bates CJ (May 1989). "Riboflavin deficiency, metabolic rate and brown adipose tissue function in sucking and weanling rats". The British Journal of Nutrition. 61 (3): 475–483. doi:10.1079/bjn19890137. PMID 2547428.
- ↑ Sebrell, W. H.; R. H. Onstott (1938). "Riboflavin Deficiency in Dogs". Public Health Reports. 53 (3): 83–94. doi:10.2307/4582435. ISSN 0094-6214. JSTOR 4582435.
- ↑ Waisman, Harry A. (1944). "Production of Riboflavin Deficiency in the Monkey.". Experimental Biology and Medicine. 55 (1): 69–71. doi:10.3181/00379727-55-14462. ISSN 1535-3702. Retrieved 2015-02-14.
- ↑ Romanoff, Alexis L.; J. C. Bauernfeind (1942). "Influence of riboflavin-deficiency in eggs on embryonic development (gallus domesticus)". The Anatomical Record. 82 (1): 11–23. doi:10.1002/ar.1090820103. ISSN 1097-0185. Retrieved 2015-02-14.
- ↑ 10. Gibson S. Rosalind, Riboflavin in Principles of Nutritional Assessment, 2nd ed. OXFORD university press, 2005
- ↑ Tilloston JA, Bashor EM. An enzymatic measurement of the riboflavin status in man. American J. Of Clin. Nutr., 1972; 72:251-261
- ↑ Bailey AL, Maisey S, Southon S, Wright AJ, Finglas PM, Fulcher RA (Feb 1997). "Relationships between micronutrient intake and biochemical indicators of nutrient adequacy in a "free-living' elderly UK population". The British Journal of Nutrition. 77 (2): 225–42. doi:10.1079/BJN19970026. PMID 9135369.
- ↑ Rutishauser IHE, Bates CJ, Paul AA, and et al. Long term vitamin status and dietary intake of health elderly subjects. I. Riboflavin. British J. of Nutr. , 1979; 42:33-42
- ↑ Gibson S. Rosalind, Riboflavin in Principles of Nutritional Assessment, 2nd ed. OXFORD university press, 2005.
- 1 2 Brody, Tom (1999). Nutritional Biochemistry. San Diego: Academic Press. ISBN 0-12-134836-9. OCLC 162571066.
- ↑ Sándor PS, Afra J, Ambrosini A, Schoenen J (Jan 2000). "Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials". Headache. 40 (1): 30–5. doi:10.1046/j.1526-4610.2000.00005.x.
- ↑ Schoenen J, Jacquy J, Lenaerts M (Feb 1998). "Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial". Neurology. 50 (2): 466–70. doi:10.1212/wnl.50.2.466. PMID 9484373.
- ↑ Gaul C, Diener HC, Danesch U (2015). "Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial". J Headache Pain. 16: 516. doi:10.1186/s10194-015-0516-6. PMC 4393401
 . PMID 25916335.
. PMID 25916335. - ↑ Bruijn J, Duivenvoorden H, Passchier J, Locher H, Dijkstra N, Arts WF (Dec 2010). "Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial". Cephalalgia. 30 (12): 1426–1434. doi:10.1177/0333102410365106. PMID 20974610.
- ↑ Maizels M, Blumenfeld A, Burchette R (Oct 2004). "A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial". Headache. 44 (9): 885–890. doi:10.1111/j.1526-4610.2004.04170.x.
- ↑ Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP (Jun 2004). "Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light". Transfusion. 44 (6): 877–885. doi:10.1111/j.1537-2995.2004.03355.x. PMID 15157255.
- ↑ Goodrich RP, et al., "The Mirasol PRT System for Pathogen Reduction of Platelets and Plasma: An Overview of Current Status and Future Trends." Transfusion and Apheresis Science 2006; 35 (1): 5-17.
- 1 2 Goodrich RP, et.al,Chapter 5:"The Antiviral and Antibacterial Properties of Riboflavin and Light: Applications to Blood Safety and Transfusion Medicine."Flavins: Photochemistry and Photobiology, Vol. 6, 2006, Royal Society of Chemistry; Cambridge, United Kingdom. E Silva and AM Edwards, editors.
- ↑ Kumar V, Lockerbie O, Keil SD, Ruane PH, Platz MS, Martin CB, Ravanat JL, Cadet J, Goodrich RP (2004). "Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level". Photochemistry and Photobiology. 80: 15–21. doi:10.1562/2003-12-23-RA-036.1. PMID 15339215.
- ↑ Hardwick CC, Herivel TR, Hernandez SC, Ruane PH, Goodrich RP (2004). "Separation, identification and quantification of riboflavin and its photoproducts in blood products using high-performance liquid chromatography with fluorescence detection: a method to support pathogen reduction technology". Photochemistry and Photobiology. 80 (3): 609–615. doi:10.1562/0031-8655(2004)080<0609:TNSIAQ>2.0.CO;2. PMID 15382964.
- ↑ Mastropasqua L (Nov 2015). "Collagen cross-linking: when and how?". Eye and Vision. 2. doi:10.1186/s40662-015-0030-6.
- 1 2 Unna, K; Greslin, JG (1942). "Studies on the toxicity and pharmacology of riboflavin". J Pharmacol Exp Ther. 76 (1): 75–80.
- ↑ Boehnke, C; Reuter, U; Flach, U; Schuh-Hofer, S; et al. (July 2004). "High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre". European Journal of Neurology. 11 (7): 475–7. doi:10.1111/j.1468-1331.2004.00813.x. PMID 15257686.
- ↑ Zempleni J, Galloway JR, McCormick DB (Jan 1996). "Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans". The American Journal of Clinical Nutrition. The American Society for Nutrition. 63 (1): 54–66. PMID 8604671.
- ↑ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- ↑ Stahmann KP, Revuelta JL, Seulberger H (May 2000). "Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production". Applied Microbiology and Biotechnology. 53 (5): 509–516. doi:10.1007/s002530051649. PMID 10855708.
- ↑ Sims GK, O'loughlin EJ (Oct 1992). "Riboflavin Production during Growth of Micrococcus luteus on Pyridine". Applied and Environmental Microbiology. 58 (10): 3423–3425. PMC 183117
 . PMID 16348793.
. PMID 16348793.
Further reading
- Schoenen J, Jacquy J, Lenaerts M (Feb 1998). "Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial". Neurology. 50 (2): 466–470. doi:10.1212/wnl.50.2.466. PMID 9484373.
External links
- National Institutes for Health, Riboflavin Fact Sheet for Health Professionals
- Higdon, Jane, "Riboflavin", Micronutrient Information Center, Linus Pauling Institute, Oregon State University
