Reverse genetics
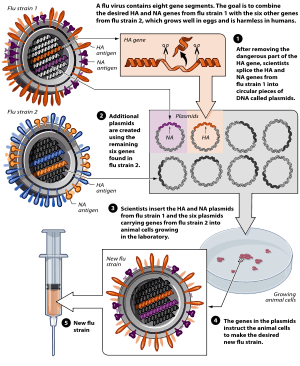
Reverse genetics is an approach to discover the function of a gene by analyzing the phenotypic effects of specific engineered gene sequences. This investigative process proceeds in the opposite direction of so-called forward genetic screens of classical genetics. Simply put, while forward genetics seeks to find the genetic basis of a phenotype or trait, reverse genetics seeks to find what phenotypes arise as a result of particular genetic sequences.
Automated DNA sequencing generates large volumes of genomic sequence data relatively rapidly. Many genetic sequences are discovered in advance of other, less easily obtained, biological information. Reverse genetics attempts to connect a given genetic sequence with specific effects on the organism.
Techniques used in reverse genetics
To learn the influence a sequence has on phenotype, or to discover its biological function, researchers can engineer a change or disruption in the DNA. After this change has been made a researcher can look for the effect of such alterations in the whole organism. There are several different methods of reverse genetics that have proved useful:
Directed deletions and point mutations
Site-directed mutagenesis is a sophisticated technique that can either change regulatory regions in the promoter of a gene or make subtle codon changes in the open reading frame to identify important amino residues for protein function.
Alternatively, the technique can be used to create null alleles so that the gene is not functional. For example, deletion of a gene by gene targeting (gene knockout) can be done in some organisms, such as yeast, mice and moss. Unique among plants, in Physcomitrella patens, gene knockout via homologous recombination to create knockout moss (see figure) is nearly as efficient as in yeast.[2] In the case of the yeast model system directed deletions have been created in every non-essential gene in the yeast genome.[3] In the case of the plant model system huge mutant libraries have been created based on gene disruption constructs.[4] In gene knock-in, the endogenous exon is replaced by an altered sequence of interest.[5]
In some cases conditional alleles can be used so that the gene has normal function until the conditional allele is activated. This might entail ‘knocking in’ recombinase sites (such as lox or frt sites) that will cause a deletion at the gene of interest when a specific recombinase (such as CRE, FLP) is induced. Cre or Flp recombinases can be induced with chemical treatments, heat shock treatments or be restricted to a specific subset of tissues.
Another technique that can be used is TILLING. This is a method that combines a standard and efficient technique of mutagenesis with a chemical mutagen such as ethyl methanesulfonate (EMS) with a sensitive DNA-screening technique that identifies point mutations in a target gene.
Gene silencing
The discovery of gene silencing using double stranded RNA, also known as RNA interference (RNAi), and the development of gene knockdown using Morpholino oligos, have made disrupting gene expression an accessible technique for many more investigators. This method is often referred to as a gene knockdown since the effects of these reagents are generally temporary, in contrast to gene knockouts which are permanent.
RNAi creates a specific knockout effect without actually mutating the DNA of interest. In C. elegans, RNAi has been used to systematically interfere with the expression of most genes in the genome. RNAi acts by directing cellular systems to degrade target messenger RNA (mRNA).
RNAi interference, specifically gene silencing, has become a useful tool to silence the expression of genes and identify and analyze their loss-of-function phenotype. When mutations occur in alleles, the function which it represents and encodes also is mutated and lost; this is generally called a loss-of-function mutation.[6] The ability to analyze the loss-of-function phenotype allows analysis of gene function when there is no access to mutant alleles.[7]
While RNA interference relies on cellular components for efficacy (e.g. the Dicer proteins, the RISC complex) a simple alternative for gene knockdown is Morpholino antisense oligos. Morpholinos bind and block access to the target mRNA without requiring the activity of cellular proteins and without necessarily accelerating mRNA degradation. Morpholinos are effective in systems ranging in complexity from cell-free translation in a test tube to in vivo studies in large animal models.
Interference using transgenes
A molecular genetic approach is the creation of transgenic organisms that overexpress a normal gene of interest. The resulting phenotype may reflect the normal function of the gene.
Alternatively it is possible to overexpress mutant forms of a gene that interfere with the normal (wildtype) gene's function. For example, over-expression of a mutant gene may result in high levels of a non-functional protein resulting in a dominant negative interaction with the wildtype protein. In this case the mutant version will out compete for the wildtype proteins partners resulting in a mutant phenotype.
Other mutant forms can result in a protein that is abnormally regulated and constitutively active (‘on’ all the time). This might be due to removing a regulatory domain or mutating a specific amino residue that is reversibly modified (by phosphorylation, methylation, or ubiquitination). Either change is critical for modulating protein function and often result in informative phenotypes.
See also
References
- ↑ Egener et al. BMC Plant Biology 2002 2:6 doi:10.1186/1471-2229-2-6
- ↑ Reski R (1998). "Physcomitrella and Arabidopsis: the David and Goliath of reverse genetics". Trends Plant Sci. 3 (6): 209–210. doi:10.1016/S1360-1385(98)01257-6.
- ↑ Winzeler, EA; et al. (1999). "Functional characterization of the Saccharomyces cervisiae genome by precise deletion and parallel analysis". Science. 285 (5429): 901–6. doi:10.1126/science.285.5429.901. PMID 10436161.
- ↑ Schween G; Egener T; Fritzkowsky D; Granado J; et al. (2005). "Large-scale analysis of 73329 Physcomitrella plants transformed with different gene disruption libraries: Production parameters and mutant phenotypes". Plant Biology. 7 (3): 228–237. doi:10.1055/s-2005-837692. PMID 15912442.
- ↑ Manis, John P., M.D. (December 13, 2007). "Knock Out, Knock In, Knock Down — Genetically Manipulated Mice and the Nobel Prize" (PDF). New England Journal of Medicine. Waltham, MA, USA: Massachusetts Medical Society. 357 (24): 2426–2429. doi:10.1056/NEJMp0707712. ISSN 1533-4406. OCLC 34945333. Retrieved December 26, 2011.
- ↑ McClean, Phillip. "Types of Mutations". Genes and Mutations. North Dakota State University. Retrieved April 27, 2015.
- ↑ Lamour, Kurt; Tierney, Melinda. "An Introduction to Reverse Genetic Tools for Investigating Gene Function". APSnet. The American Phytopathological Society.
External links
| Library resources about Reverse genetics |
- From the National Institute of Allergy and Infectious Diseases (NIAID) site:
- From the National Center for Biotechnology Information (NCBI) site:
- Neumann G; Hatta M; Kawaoka Y (2003). "Reverse genetics for the control of avian influenza". Avian Dis. 47 (3 Suppl): 882–7. doi:10.1637/0005-2086-47.s3.882. PMID 14575081.
- Neumann G; Fujii K; Kino Y; Kawaoka Y (November 2005). "An improved reverse genetics system for influenza A virus generation and its implications for vaccine production". Proc. Natl. Acad. Sci. U.S.A. 102 (46): 16825–9. doi:10.1073/pnas.0505587102. PMC 1283806
 . PMID 16267134.
. PMID 16267134. - Ozaki H; Govorkova EA; Li C; Xiong X; et al. (February 2004). "Generation of high-yielding influenza A viruses in African green monkey kidney (Vero) cells by reverse genetics". J. Virol. 78 (4): 1851–7. doi:10.1128/JVI.78.4.1851-1857.2004. PMC 369478
 . PMID 14747549.
. PMID 14747549.